Translate this page into:
An Audit of Requests for Fresh Frozen Plasma in a Tertiary Care Center in South India
Address for correspondence: Dr. Prakash H Muddegowda, E-mail: medicoprakash@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
To audit the fresh frozen plasma (FFP) usage with an insight into various guidelines.
Materials and Methods:
The blood bank records pertaining to FFP usage in patients admitted in our medical college hospital were retrospectively reviewed for 2 years for usage of FFP in various departments and evaluated for appropriateness of usage based on various guidelines, which included the 2013 guidelines published by the National Health and Medical Research Council and the Australasian Society for Blood Transfusion.
Results:
A total of 785 units of FFPs were transfused to 207 patients during the study period. The appropriate usage was found to be 59.3%, and the usage was most appropriate in massive transfusions.
Conclusion:
This study highlights the nonadherence to guidelines among clinicians which is mainly due to lack of knowledge of appropriate usage.
Keywords
Appropriate
audit
fresh frozen plasma
INTRODUCTION
The use of fresh frozen plasma (FFP) in hospital practice has risen in the past few years. The indications for transfusing FFP is limited and when transfused they can have unpredictable adverse effects. This component, which is a good source of coagulation factors, has been administered indiscriminately without scientific basis or clinical evidence. Evidence shows that the clinical use of blood between different hospitals, different specialties, and different clinical specialists, even within the same group show substantial disparities in the use of various blood components.[123]
Inappropriate use of blood components increases health expenditure, side effects, and risk of transfusion-transmitted infections. The side effects range from allergic reactions, anaphylaxis, transfusion-related acute lung injury, and hemolysis from transfused antibodies to blood group antigens, especially A and B. The usage of FFP in surgical or traumatic bleeding should be strictly guided by coagulation studies.[145]
The National Health and Medical Research Council (NHMRC) and the Australian Society for Blood Transfusion Guidelines (2013), the College of American Pathologist, and the British Committee for Standards in Haematology (2009) have published the guidelines for appropriate use of FFP, and to minimize the misuse of FFP. However, despite these guidelines, many studies from around the world still report a high frequency of inappropriate FFP usage.[1236]
Updated guidelines of NHMRC 2013 suggests avoidance of prophylactic use of FFP in cardiac surgeries or in coagulopathies, including liver disorders (if more specific therapy is available), in abnormal coagulation tests (prothrombin time [PT] and activated partial thromboplastin time [aPTT] are poorly predictive of bleeding), in immunodeficiency states, or as volume expanders, and recommends each FFP transfusion should be an independent clinical decision based on risks and benefits to patients and specialist hematology advise to be taken, when necessary.[237]
A very handful of studies, both nationally and internationally is found auditing the FFP usage. This study was conducted to audit the appropriateness of FFP usage in accordance with international guidelines in our setup.
MATERIALS AND METHODS
This retrospective study was conducted in the Department of Immunohematology and Transfusion Medicine at Vinayaka Missions Kirupananda Variyar Medical College and Hospital, Salem. The requests for FFP transfusion during the study period between January 2013 and February 2015 were analyzed for appropriateness as per international guidelines. Patients who received other supplements such as whole blood, packed cells, and Vitamin K, were excluded from the study.
Patient information collected included provisional clinical diagnosis, indication for FFP, a specialty of the requesting clinician, the demographic data including age and gender of the patient, number of units transfused and patient's pre- and post-transfusion INR if any. The usage of FFP was divided into two categories, appropriate and inappropriate, based on the guidelines of NHMRC (2013).[1678] Infusion of 10–15 ml/kg body weight of the patient was considered as adequate dose of FFP. The usage was called appropriate if it was according to NHMRC guidelines, i.e., for proper indications and was administered in an appropriate dosage.
RESULTS
A total of 785 units of FFP were transfused to 207 patients during the study period. The age of patients who received FFP transfusion ranged from 13 years to 68 years. In this study, most of the patients who received FFP transfusion were in the fourth decade [Table 1]. The department-wise collection is shown in Table 2. The appropriateness and inappropriateness of FFP are shown in Tables 3 and 4.

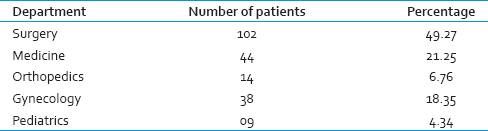
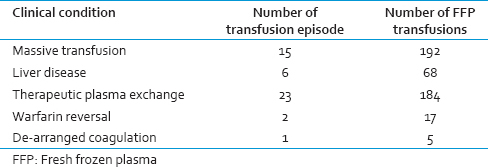
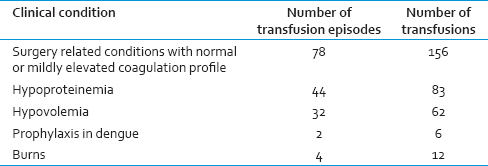
In this study, the appropriate usage was found to be 59.36%, and inappropriate usage was 40.64%. The highest rate appropriate usage was found in patients with massive transfusion. Single or double unit FFP transfusion accounts for more than 50% of inappropriate FFP transfusions while transfusions for hypoproteinemia and hypovolemia account for rest of the cases.
DISCUSSION
The use of blood and blood components has been indiscriminate due to easy availability as well as inadequate clinician knowledge of the recommended guidelines of components usage. Blood bank audits should be regularly performed to identify misuse and also as a part of quality control. The increased inappropriate use is affecting the limited resource, while impacting health care cost along with increased TTI risk.[1467]
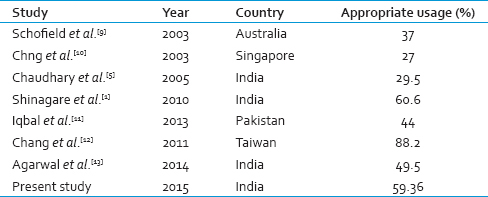
In this study, audit of 785 FFP transfusions in 207 patients identified 319 (40.64%) requests for transfusions were inappropriate. The common areas of misuse include volume replacement, mildly elevated PT, aPTT in the absence of bleeding, hypoproteinemia, postoperative wound healing, prophylactic transfusion in dengue, and other single or two-unit transfusions, which were subtherapeutic. FFP is a frequently prescribed blood product. Its use continues to rise, despite the fact that the supply of plasma derived from allogeneic blood donation is finite. Unfortunately, this product is commonly overused or inappropriately used. Comparable data have been reported at national and international level [Table 5].[15910111213]
Contrary to the belief among many clinicians, FFP transfusions are not risk-free. Allergic reaction, fluid overload, transfusion-related acute lung injury, immune suppression, hemolysis, and infectious complications can all be caused by FFP administration. It is prudent for FFP transfusion to be given only when clearly clinically indicated, so as to avoid exposing patients to unnecessary risks and higher health care costs. A study done by Sarani et al., concludes transfusion of FFP is associated with an increased risk of infection in critically ill patients.[1341415]
In our study, the major indications for FFP transfusions were used for volume expansion followed by therapeutic plasma exchange. We believe that the widespread uncertainty about the appropriate indications of FFP among the clinicians is the cause of this high rate of inappropriate FFP transfusions. In addition, due to litigation atmosphere, precaution transfusions are also known to happen. Lack of awareness about blood component usage, especially FFP usage is the most common reason for this inappropriate use of FFP.[12341112]
Optimal transfusion and following guidelines will reduce the wastage of precious products, adverse effects, and health care costs, especially in resource-poor countries with low infrastructure. Conducting repeated seminars, continuing medical education programs, and establishing hospital transfusion committees can play a vital role in improving transfusion practices and optimal use of FFP.[34]
This study identified the generalized and widespread irrational use of FFP among specialists in our medical college hospital. It is strongly recommended to establish the Hospital Transfusion Committee to give guidelines and monitor if transfusion practices adhere to these guidelines. Awareness programs regarding blood component usage in various clinical conditions should be conducted for clinicians regularly. Furthermore, it is planned that blood request forms should carry appropriate indications to remind clinicians about appropriate usage. A Computerized Transfusion Decision Support System will also help to bring down unnecessary transfusions. These practices will prevent wastage of FFP, avoid shortages in times of crisis, and reduce unwanted treatment cost.[6101112]
CONCLUSION
FFP is a precious product; we propose a preferential use of FFP for those patients who fulfill the guidelines and have a high pretransfusion INR. We feel that further studies on these lines are required to improve the utilization of this important blood product.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- An audit of fresh frozen plasma usage and effect of fresh frozen plasma on the pre-transfusion international normalized ratio. Asian J Transfus Sci. 2010;4:128-32.
- [Google Scholar]
- An audit of appropriate usage of blood products in blood bank in a Tertiary Care Hospital Rajkot. Int J Curr Res Rev. 2014;6:37-40.
- [Google Scholar]
- Utilization management in the blood transfusion service. Clin Chim Acta. 2014;427:178-82.
- [Google Scholar]
- Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133-9.
- [Google Scholar]
- Evaluation of fresh frozen plasma usage at a Tertiary Care Hospital in North India. ANZ J Surg. 2005;75:573-6.
- [Google Scholar]
- The good use of plasma. A critical analysis of five international guidelines. Blood Transfus. 2008;6:18-24.
- [Google Scholar]
- Canberra: National Health and Medical Research Council (NHMRC)/Australian Society of Blood Transfusion (ASBT) 2001. Clinical Practice Guidelines. Appropriate Use of Fresh Frozen Plasma and Cryoprecipitate; C.. :09. Available from: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp78.pdf
- [Google Scholar]
- Appropriateness of platelet, fresh frozen plasma and cryoprecipitate transfusion in New South Wales public hospitals. Med J Aust. 2003;178:117-21.
- [Google Scholar]
- An audit of fresh frozen plasma usage in an Acute General Hospital in Singapore. Singapore Med J. 2003;44:574-8.
- [Google Scholar]
- A clinical audit of fresh frozen plasma usage. J Rawalpindi Med Coll. 2013;17:122-4.
- [Google Scholar]
- The effects of a computerized transfusion decision support system on physician compliance and its appropriateness for fresh frozen plasma use in a medical center. Am J Clin Pathol. 2011;135:417-22.
- [Google Scholar]
- An audit of fresh frozen plasma usage in a Tertiary Trauma Care Centre in North India. Indian J Hematol Blood Transfus. 2014;30:328-32.
- [Google Scholar]
- Improvement in fresh frozen plasma transfusion practice: Results of an outcome audit. Transfus Med. 2004;14:231-5.
- [Google Scholar]
- Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med. 2008;36:1114-8.
- [Google Scholar]





