Translate this page into:
Appropriate Method of TIBC Estimation in Reference to Serum Transferrin Levels
Address for correspondence: Hardik Mahant, MBBS, MD, Department of Biochemistry, Medical College Baroda, Vadodara, 390001, Gujarat, India (e-mail: dr.hardikmahant@yahoo.co.in).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
The currently available various methods of estimation of total iron binding capacity (TIBC) show marked variation in reference range. Although serum transferrin (TF) level is a sensitive indicator of iron status, its measurement requires immunoassay equipment which may not be available in many routine biochemistry laboratories. So, this study was planned to find the most appropriate method to estimate TIBC.
Objectives
This study aimed to compare different methods of TIBC estimation and to corelate the TIBC values obtained by different methods with serum TF concentration.
Material and Methods
This analytical cross-sectional study was performed in the clinical chemistry laboratory of the Biochemistry Department of Medical College Baroda & SSG Hospital, Vadodara, Gujarat, on 250 leftover serum samples destined to be discarded. In all these samples, serum TIBC was estimated by direct method, indirect method, as well as calculated method (iron + unsaturated iron binding capacity [UIBC]) along with the measurement of serum TF level.
Statistical Analysis
Among the different methods, repeated analysis of variance (ANOVA) analysis and Bland–Altman plot were used to find out significance of difference. Correlation coefficients were found between different methods of TIBC estimation and serum TF levels.
Results
The means of TIBC by calculated, indirect, and direct methods were 344.51, 342.23, and 378.24 µg/dL, respectively. The mean of serum TF was 295.3 mg/dL. There was statistically significant difference between TIBC by direct and indirect methods and between direct and calculated methods. There was a strong positive correlation between TIBC by direct method and serum TF (r = 0.888, p < 0.0001), but there was moderate correlation between TIBC by indirect method and serum TF (r = 0.748, p < 0.04), and between TIBC by calculated method and serum TF (r = 0.725, p < 0.05).
Conclusion
Among different methods of estimation of TIBC, direct method is more reliable in reference to serum TF levels.
Keywords
TIBC
serum transferrin
iron status
Introduction
Iron deficiency and iron overload are quite prevalent health problems in the society and biochemical parameters can play an important role in assessing iron status and thus help in management of a patient. Biochemical parameters, like total iron-binding capacity (TIBC), serum iron, transferrin (TF), ferritin, and others, are commonly used for assessing iron status in both iron deficiency and iron overload. Iron levels alone are not very informative due to diurnal variation, alteration due to iron ingestion, interference in measurement by glassware-reagent contamination, and poor correlation with hemoglobin status. So, for definitive diagnosis, other parameters, like serum TF, TIBC, along with serum iron and the ratio of serum iron to TIBC are used.[1,2] The fraction of TF to which iron is not actually bound is known as the unsaturated iron-binding capacity (UIBC).[3] Since under physiological conditions iron is exclusively bound to β-globulin TF, determination of TF concentration in serum offers an alternative for assessing the TIBC. TIBC and UIBC are more often used in clinical practice. Since the different methods of TIBC estimation have marked variation, it is difficult to correlate them with disease status and compare the results of different laboratories.[3] Although serum TF level is a sensitive indicator of iron status, its estimation requires special equipment and is expensive. In this scenario, current study was planned to find a method of determination of TIBC which is reliable and easily applicable in clinical laboratory and can be used as an indicator of iron status in reference to TF levels.
Materials and Methods
This analytical cross-sectional study was performed in the Clinical Chemistry Laboratory at SSG Hospital attached to the Medical College Baroda in Vadodara, Gujarat. Approval and ethical clearance were obtained from concerned Institutional Ethics Committee for Human Research.
Nonlipemic, nonicteric, and nonhemolyzed left over serum samples that are destined for discard in which routine biochemical parameters had already been tested were included in the study. Since lipemia, bilirubin, and hemolysis can interfere with laboratory estimation of various parameters, only samples with mentioned criteria were included.
The samples which were outside the reference range for protein, albumin, urea and creatinine and samples of patients in intensive care units (ICUs) or labor room were not included in the study because TF is an acute phase protein, its levels are altered in many clinical conditions and do not give true picture of iron status in such situations. Out of 1,518 samples screened, 250 samples which fulfilled these criteria included in the study.
Serum iron, serum UIBC, serum TIBC by indirect method, serum TIBC by direct method, and serum TF levels were estimated on all these samples. The serum iron and serum UIBC were estimated by colorimetric end-point method using semi autoanalyzer (ERBA CHEM-V7). Indirect TIBC was estimated by saturation-precipitation followed by colorimetric end-point method using semiautoanalyzer (ERBA CHEM-V7). Direct TIBC was measured by Colorimetric method using fully autoanalyzer (Transasia ERBA EM-320). TIBC by calculated method was found by formula: TIBC (calculated) = serum iron + serum UIBC. TF was measured by the Sandwich Elisa method using ELISA reader (Alere) and washer (Microlab). All the results were entered in an Excel sheet. The Normality of the data was checked by Shapiro–Wilk test. Data were expressed as mean and standard deviation (SD) for iron, UIBC, TIBC, and TF. Out of 250 samples, two results of TIBC by direct method were unexpectedly too far from the mean, and so they were excluded. In data of 248 samples, statistical analysis was done by using repeated measures analysis of variance (ANOVA) and Bland–Altman plot to find out significance of difference. Pearson's correlation coefficient was applied to find different methods of TIBC estimation and serum TF levels. Also, r greater than 0.60 to 0.80 was considered moderately strong correlation, while r greater than 0.80 was considered strong correlation. A p-value of less than 0.05 was considered significant. All statistical analysis was done using MedCalc statistical software version 12.5.0.
Results and Analysis
Out of 248 samples included in our study, 133 samples were of males and 115 samples were of females, the mean age for males and female was 40.71 ± 13.62 and 37.3 ± 11.96 years, respectively (p > 0.05).
►Table 1 shows levels of serum iron, serum UIBC, serum TF, and TIBC by calculated, indirect, and direct methods. The mean of calculated, indirect, and direct methods of TIBC were 344.51, 342.20, and 378.20 µg/dL, respectively.
| Parameter | Mean ± SD | Range |
|---|---|---|
| Serum iron (µg/dL) | 115.3 ± 32.07 | 31–215 |
| Serum UIBC (µg/dL) | 220.25 ± 110.37 | 40–613 |
| Serum TIBC by calculated method (µg/dL) | 344.51 ± 89.96 | 146–654 |
| Serum TIBC by indirect method (µg/dL) | 342.20 ± 88.32 | 97–589 |
| Serum TIBC by direct method (µg/dL) | 378.20 ± 81.85 | 148–641 |
| Serum transferrin (mg/dL) | 295.3 ± 62.73 | 112–515 |
Abbreviations: SD, standard deviation; TIBC, total iron binding capacity; UIBC, unsaturated iron binding capacity.
►Table 2 shows comparison of TIBC by calculated, indirect, and direct methods using ANOVA test. There was a mean difference of 33.72 µg/dL between TIBC by direct method and TIBC by calculated. (p < 0.0001). Between direct and indirect methods, the mean difference was 36.01 µg/dL (p < 0.0001), while the difference between indirect and calculated methods was only 2.28 µg/dL (p > 0.05, statistically not significant)
| Factors | Mean difference | p-Value | |
|---|---|---|---|
| TIBC by calculated method | TIBC by indirect method | 2.28 | 1.0000 |
| TIBC by direct method | −33.72 | < 0.0001 | |
| TIBC by indirect method | TIBC by calculated method | −2.28 | 1.0000 |
| TIBC by direct method | −36.01 | < 0.0001 | |
| TIBC by direct method | TIBC by calculated method | 33.72 | < 0.0001 |
| TIBC by indirect method | 36.01 | < 0.0001 | |
Abbreviations: ANOVA, analysis of variance; TIBC, total iron binding capacity;
TIBC values by all these three methods were correlated with serum TF levels.
►Fig. 1A shows correlation between serum TF and calculated method of TIBC. An r-value of 0.725 with confidence interval of 0.660 to 0.779 showed moderately strong positive correlation (p < 0.05).

- (A) Correlation of TIBC (calculated) method and serum transferrin. (B) Correlation of TIBC by Indirect method and serum transferrin. (C) Correlation of TIBC by direct method and serum transferrin. TIBC, total iron binding capacity.
►Fig. 1B shows the correlation between serum TF and indirect method of TIBC. An r-value of 0.748 with confidence interval 0.688 to 0.799 showed moderately strong positive correlation (p < 0.001).
►Fig. 1C shows the r-value of 0.888 with confidence interval of 0.858 to 0.912, showing strong positive correlation between levels of TIBC by direct and serum TF (p < 0.001).
In our study, we found that TIBC by direct has a better correlation with serum TF concentration, we have compared other two methods against TIBC-direct method using Bland–Altman plot.
►Fig. 2 shows there was systematic difference between the TIBC by direct method and the TIBC by calculated method with difference of mean equal to 33.7 µg/dL. The limit of agreement was (136.2, −68.8) indicating a wide confidence interval. Although only 3.62% of the points (9 out of 248) were outside of the 95% limit of agreement, wide confidence interval shows low level of agreement between two methods
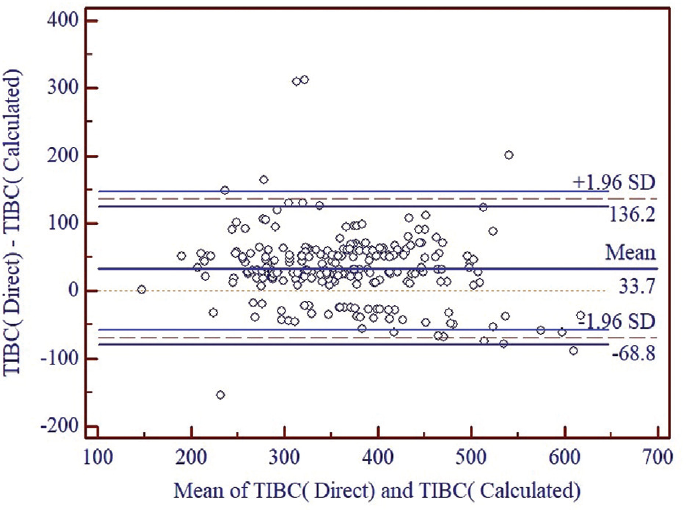
- Comparison of TIBC by direct method with TIBC by calculated method. SD, standard deviation; TIBC, total iron binding capacity.
►Fig. 3 shows that there was systematic difference between the TIBC by direct method and the TIBC by indirect method with difference of mean equal to 36.0 µg/dL. The limit of agreement was (126.7, −54.7), indicating a wide confidence interval. Although only 3.22% of the points (8 out of 248) were outside of the 95% limit of agreement, wide confidence interval shows low level of agreement between two methods.
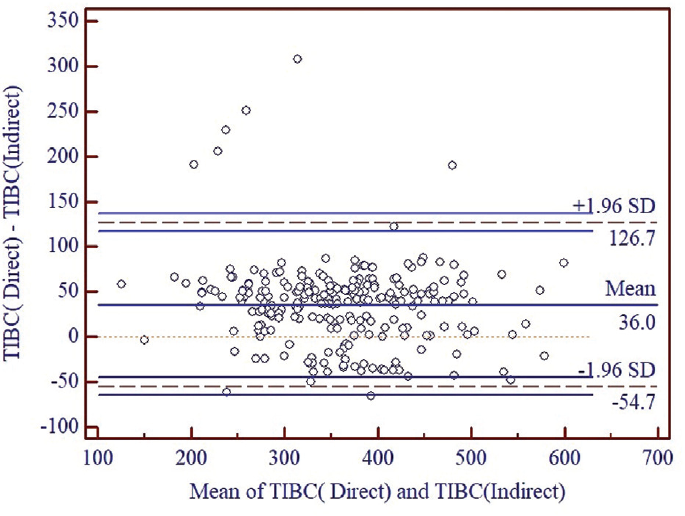
- Comparison of TIBC by direct method with TIBC by indirect method. TIBC, total iron binding capacity.
So, as shown in ►Figs. 2 and ►3, Direct method of TIBC estimation is not analytically replaceable by calculated or indirect method of TIBC estimation.
Discussion
TIBC is an indicator of maximum amount of iron needed to saturate the plasma or serum TF which is the primary iron transport protein. Serum TIBC has different methods of measurements and each has its own analytical characteristics, and there is no harmonization between methods used by different laboratories.[4] TF, an important protein for the transport of iron molecule, is used as a specific marker of iron homeostasis. With reference to the structure of TF, one mole of TF can bind with two moles of iron at two affinity binding sites for ferric iron. So, it is assumed that the TIBC correlates with serum TF levels. The techniques and instrumentation of TF measurement require special attention for good results.
Overall, laboratory assessment of iron status relies on combination of biochemical parameters, but there is a need to link these indicators to reach a meaningful clinical correlation. In this study, we measured and compared TIBC by various methods and correlated them with serum TF levels in normal healthy patients.
In this study of 248 samples, the mean ± SD for serum TIBC by calculated method, TIBC by indirect method, and TIBC by direct method were 344.51 ± 89.96 (146–654), 342.20 ± 88.32 (97–589), and 378.20 ± 81.85 (148.30–640.90) µg/dL, respectively. We used the Ferene dye for UIBC estimation and Ferrozine dye in TIBC by indirect method.
On repeated measures of ANOVA, on pair wise comparison, it was found that there was a statistically significant difference between TIBC by direct method and TIBC by calculated method (33.72) and between TIBC by direct method and TIBC by indirect method (36.01; p < 0.0001).
In a study carried by Lee,[5] TIBC by calculated method using bathophenanthroline as a chromogen was reported as 314.66 ± 41.83 (157-637) µg/dL. In the study of Yamanishi et al[6] (n = 188), the level of TIBC by calculated method was 317.32 ± 73.74 µg/dL, TIBC by indirect method using ferrozine as chromogen was 315.08 ± 74.30 µg/dL, and TIBC by direct method was 356.42 ± 82.12 µg/dL. TIBC in our study are similar to those reported in literature. They also concluded that there was statistically significant difference between values of TIBC by direct method and the values of TIBC by calculated or TIBC by indirect method. (p < 0.001). Our study is in agreement.
In our study, we found no difference between TIBC by calculated method and TIBC by indirect method. Similar conclusions have been drawn by other researchers such as Tsung et al in 1975,[7] using a radio isotope of iron and Blanck et al[8] in 2003. Our study is in agreement with these studies by almost similar results and no significant statistical difference.
Increase in UIBC values can be due to release of iron bound to TF during the process of estimation. This might be the reason for a small increase in values of calculated method of TIBC than the Indirect TIBC levels in our study.
On repeated measures of ANOVA, on pair wise comparison, it was found that there was a statistically significant difference between TIBC by Direct method and TIBC by Calculated method (33.72) and between TIBC by Direct method and TIBC by Indirect method. (36.01; p < 0.0001).
The reason for lower values in TIBC by calculated and TIBC by indirect method may be the desaturation and saturation procedures during measurement. As binding of iron to TF is not an instantaneous process. It might take some time for completion of saturation step. The time allotted to the procedure might be insufficient leading to decreased calculated TIBC/indirect TIBC.
This clearly indicates that there is substantial difference between TIBC by different methods which can further add to clinicians' dilemma, and there is need to find out a reliable method for measurement of TIBC. So different levels of TIBC by three methods were correlated with serum TF levels. The mean ± SD for serum TF levels was 295.3 ± 62.73 (112–515) mg/dL.
In correlation between value of TIBC by calculated method and serum TF level, the r-value was 0.725 (0.660–0.779) with statistically significant p-value (< 0.0001). Between indirect TIBC method and serum TF level, the r-value was 0.748 (0.688–0.799) This indicates the almost similar correlation found with TF in calculated and indirect methods of TIBC measurement. In study of Yamanishi et al,[6] correlation between indirect method using ferrozine as chromogen and TF levels, r-value was 0.973.
In this study, the correlation between TIBC by direct method and serum TF level, the r-value was 0.888 (0.858–0.912) with a significant p-value. This shows that the TIBC by direct method has better correlation with serum TF levels. It is important to note that TIBC by direct method has the narrow confidence interval in correlation to TF levels than the other two methods. Better results were found by Yamanishi et al[6] with r-value of 0.983 between direct TIBC by direct method and serum TF.
As the TIBC by direct method has the better correlation with the TF, the Bland–Altman plot was used to compare between TIBC by direct method and TIBC by calculated method and between TIBC by direct method and TIBC by indirect method. We found that the mean difference between direct and calculated method was 33.7 µg/dL with wide limit of agreement (136.6 to −68.8 µg/dL). Between direct and indirect methods, the mean difference was 36.0 µg/dL with wide limit of agreement (123.7 to −54.7 µg/dL).
In this study, neither calculated method nor indirect method are analytically replaceable with direct method due to wide limit of agreement and mean difference of 33.7 and 36.0 µg/dL, respectively.
TIBC by calculated method depends on levels of serum iron and UIBC. Serum iron has diurnal variation and fluctuations which are further increased during inflammatory states. Serum UIBC can be lower detected when the TF binds with HCO3− as a coligand in the presence of oxygen which affect the kinetics of the binding process. Automated process has more comfort with UIBC, but its measurement precision is better at higher concentration as in iron deficiency states. It is not a reliable indicator in iron overload conditions. So, TIBC by calculated method, based on serum iron and UIBC, does not fit to be reliable method of iron status. Indirect method of TIBC is affected by the concentrations of iron used to saturate TF in the initial steps and chemicals used during the process. The tedious two-step procedure has more chances of errors limiting its accuracy and precision. TIBC by direct dye binding method has an advantage of easy automation.[9-11]
As per our knowledge, this is the first of its kind study done to compare different methods of TIBC and to find the correlation with serum TF levels on Indian population. No other study has been found using all the three different methods of TIBC estimation and their correlation with serum TF done by Immunoassay method using ELISA. The strength of our study was that the samples selected by us represented wide range of age and included both sexes. We avoided any sample which could have altered TF levels due to any associated clinical condition and thus represent true iron status.
The limitation of our study was that there was no direct contact with the patient. The study was performed in left over sera already tested for other parameters. All samples included in study were within reference range. We have not compared at lower and higher levels, so cannot comment on lower detection limit and linearity of various methods. The other parameters like serum ferritin, serum hepcidin, etc, were not included.
This study should be performed further in future with a greater number of samples and including other iron indicators, like serum ferritin, hepcidin, carbohydrate-deficient TF (CDT)) level, TF receptors, and others to have a better insight into biochemical parameters of iron status. Future studies should be directed toward finding of specific reference intervals for different age groups, as well as for specific genetic disorders involving proteins of iron metabolism.
Conclusion
The estimation of TIBC by calculated method and indirect method cannot replace TIBC by direct method analytically. Although indirect and calculated methods provide almost similar values, these values are significantly lower compared with direct method of TIBC. Direct method corelates better with serum TF levels compared with other two methods. We conclude that TIBC by direct method has the advantage of having better correlation with serum TF level and easier application on automated analyzers.
Authors' Contributions
H.M. contributed to planning, sample collection and processing, results and analysis, and writing draft of manuscript. S.J. contributed to planning, guidance, ethical clearance, drafting of manuscript, and review of manuscript. A.P. contributed to analysis of results, statistical review, and review of manuscript. B.L. contributed to sample collection and processing, results, and analysis.
Conflict of Interest
None declared.
Funding
None.
References
- Biochemical aspects of haematology. In: Burtis CA, Ashwood ER, eds. Tietz Textbook of Clinical Chemistry (2nd). Philadelphia, PA: WB Saunders; 1994. p. :1974-2072.
- [Google Scholar]
- Plasma iron and transferrin iron-binding capacity evaluated by colorimetric and immunoprecipitation methods. Clin Chem. 1987;33(2, pt. 1):273-277.
- [CrossRef] [PubMed] [Google Scholar]
- Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin Chem Lab Med. 2002;40(10):1014-1018.
- [CrossRef] [Google Scholar]
- Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106(Suppl. 06):1606S-1614S.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of serum iron, total iron binding capacity and haemoglobin A1c level according to obesity in south Korean adults. Int J Appl Engineer Res. 2017;15:830-837.
- [Google Scholar]
- Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity. Clin Chem. 2003;49(01):175-178.
- [CrossRef] [PubMed] [Google Scholar]
- Immunological measurement of transferrin compared with chemical measurement of total iron-binding capacity. Clin Chem. 1975;21(08):1063-1066.
- [CrossRef] [PubMed] [Google Scholar]
- Serum iron and iron-binding capacity: a round-robin interlaboratory comparison study. Clin Chem. 2003;49(10):1672-1675.
- [CrossRef] [PubMed] [Google Scholar]
- Fully automated measurement of total iron-binding capacity in serum. Clin Chem. 1997;43(12):2413-2417.
- [CrossRef] [PubMed] [Google Scholar]
- Direct serum total iron-binding capacity assay suitable for automated analyzers. Clin Chem. 2002;48(01):161-166.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of precipitation reference method with new direct assay of total iron binding capacity (TIBC) JNKUMS. 2018;9(04):34-41.
- [Google Scholar]






