Translate this page into:
Role of multiplex polymerase chain reaction in diagnosing tubercular meningitis
Address for correspondence: Dr. Kiran Chawla, Profesor and Head of Microbiology, Kasturba Medical College, Manipal University, Manipal, Karnataka, India. E-mail: kiran.chawla@manipal.edu
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tuberculous meningitis (TBM) is one of the most serious manifestations of extrapulmonary tuberculosis. Timely and accurate diagnosis provides a favorable prognosis in patients with TBM. The study evaluated the use of multiplex polymerase chain reaction (PCR) in the diagnosis of TBM. A study was conducted on 74 patients clinically suspected with TBM. The cerebrospinal fluid (CSF) specimens were processed for smear microscopy, middle brook 7H9 culture, and multiplex PCR using primers directed against IS6110 gene and 38 kD protein for detection of Mycobacterium tuberculosis. The results were analyzed to assess the role of multiplex PCR in the diagnosis of TBM. A total of 26 (35.1%) patients were diagnosed with TBM. Microscopy was negative in all while culture was positive in two cases only. Comparing with clinical diagnosis and CSF adenosine deaminase levels of ≥10 U/L, multiplex PCR showed sensitivity, specificity, positive predictive value, and negative predictive value of 71.4%, 89.6%, 83.3%, and 81.2%, respectively, in the diagnosis of TBM.
Keywords
Culture
microscopy
polymerase chain reaction
tubercular meningitis
Tuberculosis (TB) is one of the major infectious causes of morbidity and mortality worldwide. Annually 1.8 million new cases of TB are seen in India, which accounts for one-fifth of new cases in the world, greater than in any other country.[1]
Central nervous system (CNS) TB accounts for about 5% of all extrapulmonary TB, and tuberculous meningitis (TBM) is the most serious complication.[2] Nonspecific clinical presentation of TBM makes the diagnosis often difficult, and its paucibacillary nature has led researchers to develop newer methods of diagnosis. Conventional methods of TB diagnosis like smear microscopy and culture require 104–106 and 101–102 bacilli/mL of sample respectively, to give a positive result and are time-consuming.[3] Although confirmatory diagnosis of TBM can only be done by culturing the etiological agent, it still needs 101–102 bacilli/ mL of sample for the diagnostic yield and requires 2–4 weeks for the growth of Mycobacterium tuberculosis. Hence, a diagnostic method that has shorter turn-around time and at the same time has high sensitivity and specificity is therefore desirable.
Nucleic acid amplification tests represent a major advance in the diagnosis of TB. Various targets have been used for detecting mycobacterial DNA such as 65-KD heat shock protein, ribosomal RNA, and MPB64.[4] IS6110 has been proved to be a good target because of the presence of multiple copies of this insertion sequence (1–20) in most strains of M. tuberculosis complex.[5] However, the presence of occasional M. tuberculosis strains from India which lack IS6110 implies that relying only on IS6110-based polymerase chain reaction (PCR) is not advisable. In view of this, a study was planned to assess multiplex PCR using primers directed against IS6110 gene and 38 kD protein (specific for M. tuberculosis but less explored by others) for the detection of M. tuberculosis in cerebrospinal fluid (CSF) specimens in patients suspected with TBM.
A prospective study was conducted in a tertiary care center of South-Coastal Karnataka between August 2012 and July 2014. A total of 74 CSF samples from suspected cases of tubercular meningitis who were >15 years of age were included in the study and were processed for smear microscopy for acid-fast bacilli (AFB), culture and PCR for M. tuberculosis in the microbiology laboratory. Institutional Ethics Committee Clearance was obtained for conducting this study. The relevant clinical details of these patients were collected from case records. Diagnosis of TBM was made based on the following major and minor criteria as described by Kulkarni et al.[6]
Major criteria
(a) Clinically suspected patients with abnormal neurological signs and symptoms, for example, fever, neck stiffness, altered sensorium, and convulsions. (b) Positive CSF cytology and biochemical findings; pleocytosis (>10 leukocytes/mL), elevated protein concentration (>40 mg/dL), CSF glucose <60% of serum glucose and (c) Microbiologically positive (Ziehl–Neelsen smear positive for AFB or culture positive for M. tuberculosis and negative for other bacteria).
Minor criteria
(1) Close contact with a known case of active TB, (2) positive Mantoux skin test, (3) radiographic evidence of active TB at the extraneural site, (4) abnormal findings in computed tomography, and (5) clinical response to antitubercular drugs.
Based on above criteria, the patients were divided into three groups:
Group 1 (Definitive TBM group): Fulfilled major criteria A and B and any two of the minor criteria. Group 2 (Probable TBM group): Clinically suspected but do not fulfill two major and two minor criteria. Group 3 (Control group): Cases diagnosed with neurological pathology other than TBM.
All CSF samples were centrifuged at 3000 rpm for 5 min. Deposit was divided into three parts and subjected to smear microscopy by Ziehl–Neelsen staining, Middle Brook 7H9 broth culture, and multiplex PCR as per the standard guidelines. PCR was standardized with a standard strain of M. tuberculosis (H37Rv) which was found to have a sensitivity to detect the MTB DNA equivalent to 2–3 organisms. DNA extraction was done using commercial extraction kit as per manufacturer's (Qiagen, Germany) instructions. In each independent PCR assay, positive control and negative control were included. The positive control was the DNA of M. tuberculosis (H37Rv) and negative control included the PCR grade water. Identification of M. tuberculosis was done using primers designed to amplify 38 KD and IS6110 gene in the M. tuberculosis complex, and the expected target size was 340 bp and 245 bp, respectively. The sequence of primers used were KD1 (5'- CCA AGC AAG ATC CCG AGG GCT – 3'); KD2 (5’ – TTG ATG ATC GGG TAG CCG TCC 3') for 38 kDa gene (340 bp) and INS1 (5’ CGT GAG GGC ATC GAG GTG GC 3'); INS2 (5’ GCG TAG GCG TCG GTG ACA AA 3') for IS6110 gene (245 bp).[7]
In a 25 µl reaction, multiplex PCR was done for forty cycles including denaturation at 94°C for 1 min, annealing of primers at 65°C for 1 min and primer extension at 72°C for 1 min and one cycle of final extension at 72°C for 10 min. The amplification products were separated on 2% agarose gel and samples showing either the presence of both 340 and 245 bp bands or any one of the two bands under ultraviolet transilluminator (Vilber Lourmat, France) were considered positive [Figure 1]. The sensitivity, specificity, positive predictive, and negative predictive values (NPVs) of multiplex PCR were calculated.
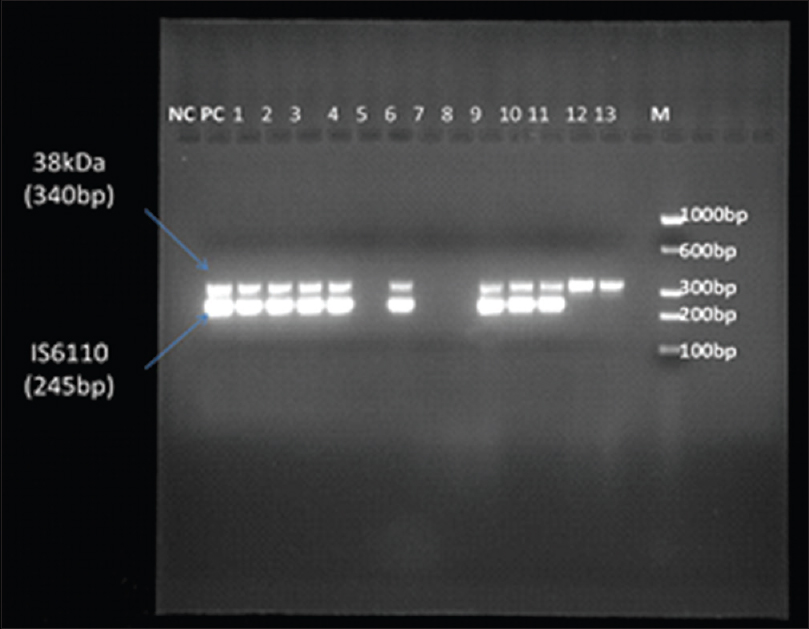
- Gel photograph showing the result of multiplex polymerase chain reaction. Multiplex polymerase chain reaction with 38 kDa (340 bp) and IS6110 (245 bp) genes of Mycobacterium tuberculosis in cerebrospinal fluid samples. Lane M = Molecular marker, PC = axControl DNA of H37RV and 1–13 are sample
As per the major and minor criteria, 26 (35.1%) patients were diagnosed with definitive TBM and 24 (32.4%) with probable TBM. Among patients with definitive TBM, 16 were positive by multiplex PCR (IS6110 and 38 kD) and M. tuberculosis was grown in two patients but AFB were not seen in any of the samples by microscopy. The samples (n = 2) showing growth of M. tuberculosis were also positive by PCR. Positivity of multiplex PCR was 61.5% and 29.2%, respectively, among definitive and probable group. Two of the 16 cases positive by PCR were negative for IS6110. Of the 26 definitive cases of TBM, radiological investigations suggestive of disease were observed in 15 (57.7%) patients.
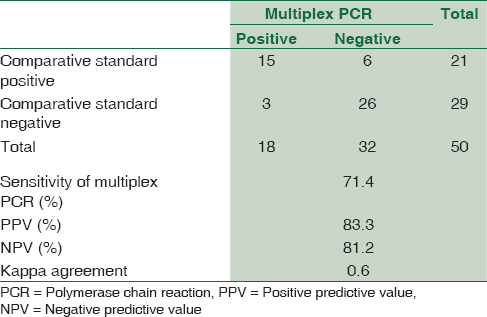
Due to low culture positivity observed in the study, the results of multiplex PCR were compared with combination of clinical diagnosis and CSF adenosine deaminase (ADA) level of ≥10 U/L (considered as comparative standard).[8] Statistical analysis revealed sensitivity, specificity, positive predictive value, and NPV of 71.4%, 89.6%, 83.3%, and 81.2% [Table 1] and kappa coefficient of agreement as 0.6 for the multiplex PCR, respectively. Multiplex PCR detected two cases extra in TBM group.
Although microscopy is very economical, it has the limitation of low sensitivity in extrapulmonary paucibacillary TB such as TBM. In the present study, all cases were AFB smear negative. The number of bacilli required for positive acid-fast staining is 104/mL. Previous studies have reported sensitivity in the range of 0–10 for smear microscopy in TBM.[69] A recent study established that both CSF volume and duration of the microscopic evaluation are independently associated with bacteriological confirmation of CNS TB suggesting that a minimum of 6 mL of CSF fluid should be examined microscopically for a period of 30 min,[9] but invariably the laboratory receives only 1–2 mL of CSF.
In the present study, culture showed positivity of 8% only among the definitive TBM group. Other case series have also reported similar low CSF culture sensitivities varying from 4.3 to 48.9%.[610] Advanced molecular methods such as PCR is considered the most sensitive and specific diagnostic method, especially in cases where clinical suspicion is high but AFB staining is negative.[11] Panagariya et al. have reported sensitivity rates of PCR ranging from 65% to 85%.[12] The sensitivity of multiplex PCR in our study was 71.4%. The importance of multiplex PCR is shown earlier by Chauhan et al.[13] Twenty percent of strains showed low copy number, whereas 11% isolates lacked IS6110 element. Our PCR primers were directed against IS6110 gene and 38 kD protein for the detection of M. tuberculosis in the CSF. There were 2 (8%) samples in our study which showed the absence of IS6110 and presence of 38 kD amplicon in multiplex PCR indicating the presence of M. tuberculosis strains lacking IS6110. A similar study done by Kulkarni et al.[7] showed that there were 4 (6.25%) strains out of 64 samples which lacked IS6110 element. PCR was negative in ten cases among the definite TBM cases (n, 26) but all these patients responded to antitubercular therapy. The various reasons for PCR negativity in these cases could be attributed to small volume of CSF received in the laboratory, low number of bacteria in the CSF, poor lysis of bacteria in the CSF samples, or the presence of some PCR inhibitors.
In probable TBM group (n = 24), seven cases were PCR positive. Among them, four patients were planned for antitubercular therapy by the clinician based on PCR results and their clinical presentation, but two patients took discharge against medical advice because of financial constraint (one patient was HIV positive and another patient had higher ADA level of 19 U/L). These four cases can be considered as TBM because their clinical condition was not improving with other line of treatment. False positivity with PCR was observed in three cases as two were diagnosed with leptospirosis and one was diagnosed with scrub typhus. False positive results in PCR could be due to contamination during sample processing or amplicon contamination. To conclude, multiplex PCR for IS6110 and 38 kD is an effective tool for early diagnosis of TBM for prompt management of TBM cases.
Limitation of our study was not using MGIT or BACTEC culture for CSF samples which could have given better culture positivity. Another limitation could be inadequate amount of CSF samples.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Evaluation of polymerase chain reaction using protein b primers for rapid diagnosis of tuberculous meningitis. Neurol India. 2010;58:727-31.
- [Google Scholar]
- Molecular detection of Mycobacterium tuberculosis in tissues showing granulomatous inflammation without demonstrable acid-fast Bacilli. Diagn Mol Pathol. 2000;9:67-74.
- [Google Scholar]
- Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: Recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406-9.
- [Google Scholar]
- Evaluation of an in-house-developed PCR for the diagnosis of tuberculous meningitis in Indian children. J Med Microbiol. 2005;54(Pt 4):369-73.
- [Google Scholar]
- An in-house multiplex PCR test for the detection of Mycobacterium tuberculosis, its validation and comparison with a single target TB-PCR kit. Indian J Med Res. 2012;135:788-94.
- [Google Scholar]
- Adenosine deaminase levels in cerebrospinal fluid as a diagnostic test for tuberculous meningitis in children. Indian J Clin Biochem. 2004;19:5-9.
- [Google Scholar]
- Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol. 2004;42:378-9.
- [Google Scholar]
- Comparison of conventional diagnostic modalities, BACTEC culture with polymerase chain reaction for diagnosis of extra-pulmonary tuberculosis. J Med Allied Sci. 2013;3:53-8.
- [Google Scholar]
- Development of a new sensitive and efficient multiplex polymerase chain reaction (PCR) for identification and differentiation of different mycobacterial species. Trop Med Int Health. 2003;8:150-7.
- [Google Scholar]
- Clinicodiagnostic features of tuberculous meningitis and the role of CSF PCR in early diagnosis: A study from North-West India. J Indian Med Assoc. 2013;111:309-12, 314.
- [Google Scholar]
- Molecular typing of Mycobacterium tuberculosis isolates from different parts of India based on IS6110 element polymorphism using RFLP analysis. Indian J Med Res. 2007;125:577-81.
- [Google Scholar]





