Translate this page into:
Spectrum of meningioma with special reference to prognostic utility of ER,PR and Ki67 expression
Address for correspondence: Dr. Chhanda Das, 31 Eastern Park, Santoshpur, Kolkata - 700 075, West Bengal, India. E-mail: chhhdas@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Meningiomas are the most common primary central nervous system neoplasms originating from the arachnoid cap cells and constitute between 13% and 26% of all intracranial tumors.
AIMS AND OBJECTIVES:
The aim of the study was to analyze the age-, sex-, and site-wise distribution of different histological patterns of meningiomas seen in our center and to assess the status of estrogen receptor (ER), progesterone receptor (PR), and proliferation marker Ki-67 in various grades of meningioma.
MATERIALS AND METHODS:
A prospective study was done in 90 cases. Patients presented with symptoms of headache and seizure and underwent subsequent excision surgery at Neurosurgery Department were taken. We have studied histological typing and grading of the tumors, and immunohistochemical staining was done for ER, PR, and Ki-67.
STATISTICAL ANALYSIS:
Two-group comparison was done using Mann–Whitney U-test and Fisher's exact test. Comparison of Ki-67 expression between Grade 1 and Grade 2 meningiomas was determined using Mann–Whitney U-test. Comparison of ER and PR status between different histological grades was done by Fisher's exact test. Two-tailed P < 0.001 was considered statistically significant.
RESULTS:
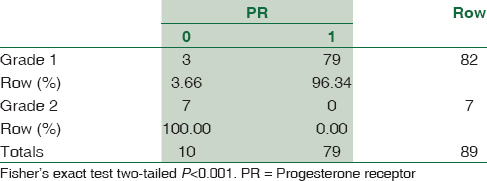
According to histological type, meningothelial meningioma is most common (38.8%) followed by transitional (22.2%). PR positivity is seen in 96.34% of Grade 1 tumors, and all Grade 2 tumors were PR negative (Fisher's exact test P < 0.001). About 3.66% of Grade 1 and all Grade 2 tumors were positive for ER (Fisher's exact test two-tailed P < 0.001). Mean Ki-67 labeling index (LI) was 2.57 ± 1.674 among Grade I tumors, 7.11 ± 1.084 in Grade II meningiomas.
CONCLUSIONS:
Most of Grade 1 meningiomas show PRs positivity and lack of ERs positivity. Meningiomas with higher proliferation index and negative PR are very likely to be Grade II or Grade III. Evaluation of ER, PR status, and Ki-67 labeling index (LI) with histological evaluation helps us in providing information about the biologic behavior of meningiomas.
Keywords
Hormone receptors
meningioma
proliferative index
Introduction
Meningiomas are the most common primary central nervous neoplasms originating from the arachnoid cap cells and constitute between 13% and 26% of all intracranial tumors.[1] Most meningiomas are slow growing and histologically benign.[2] Certain histological subtypes are associated with less favorable clinical outcomes and correspond to WHO Grades II (atypical) and III (anaplastic or malignant). Atypical meningiomas have been reported up to 20%; anaplastic (malignant) meningiomas account for between 1.0% and 2.8%.[3] Meningiomas occur most commonly in middle-aged patients, with a peak during the sixth and seventh decades. The female:male ratio being approximately 1.7:1.[4] The ratio peaks at 3.5:1 in the 40–44-year-old patients.[5] Atypical and particularly anaplastic meningiomas show a male predominance.[6] The major prognostic factor in meningiomas is the prediction of recurrence. This depends on the extent of resection, its histopathological type, grading, proliferation indices, and progesterone receptor (PR) status. High mitotic index is considered to be a strong indicator of tumor recurrence.[7] The preponderance of PRs and the scarcity of estrogen receptors (ERs) in meningiomas are well known. The aim of this study is to investigate the correlation between various grades of meningiomas with proliferation index Ki-67 and hormonal (progesterone and estrogen) markers.
Materials and Methods
The study was conducted in the Department of Pathology in association with Department of Neurosurgery, at Institute of post graduate medical education and research (I.P.G.M.E.&R) Hospital, Kolkata, from June 2013 to March 2016. Patients presenting with symptoms of headache and seizure and diagnosed radiologically as intracranial space-occupying lesions underwent subsequent excision at Neurosurgery Department were taken. The study was performed after obtaining the approval from Ethical Committee, and a total of 90 cases was selected. Hematoxylin and eosin staining was done for histological typing and grading of the tumors. Tumors were graded according to the WHO grading system. Immunohistochemical staining was done for ER, PR, and Ki-67. It was performed on 4–5 μm thick, formalin-fixed, paraffin-embedded tissue sections that were mounted on poly-L-lysine precoated slides. Paraffin wax sections were dewaxed and stained with monoclonal mouse antihuman PR 1A6 purchased from Dako (Copenhagen, Denmark), anti-ER mouse monoclonal antibodies (Dako, ERM 7047), and Ki-67 (Dako, Copenhagen, Denmark). Negative controls were run at each staining session. Positive controls were specimens using breast cancer tissue for ER, PR, and lymph node germinal center for Ki-67. All slides were examined for positively stained tumor cell nuclei. For ER and PR, the immunostained sections were graded semiquantitatively for intensity and extent of staining. Tumors with strong staining in at least 10% of nuclei or moderate staining in about 50% of nuclei were considered positive.[8] In case of Ki-67 staining, proliferative index (PI) was expressed as a percentage of positively stained cells out of 1000 tumor cells counted in the most mitotically active areas. Comparison of Ki-67 expression between Grade 1 and Grade 2 meningiomas was determined using Mann–Whitney U-test. Comparison of ER and PR status between different histological grades was done by Fisher's exact test. Two-tailed P < 0.001 was considered statistically significant.
Results
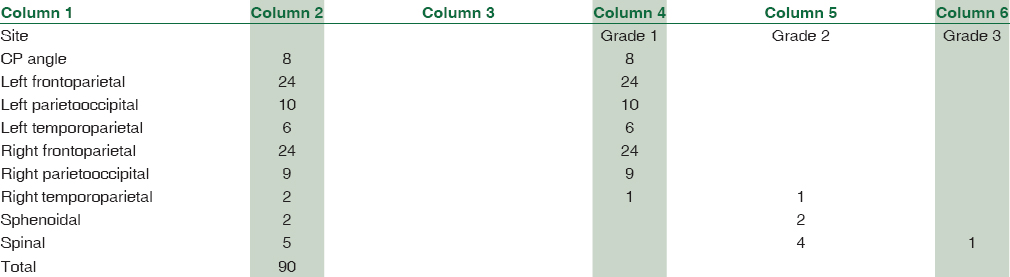
A total of 90 cases were studied over a period of 3 years. Age ranged from 6 months to 67 years (median: 44 years). The mean age (±standard deviation) of the patients was 42.29 ± 15.95. There were 62 female patients and 28 male patients. Female:male ratio was 2.2:1. According to location, 75 (83.33%) were cerebral, 8 (8.89%) were at CP angle, 2 (2.22%) were sphenoidal, and 5 (5.56%) were spinal meningiomas. According to histological type, 35 were meningothelial (38.89%), 18 fibroblastic (20%), 20 transitional (22.22%), five psammomatous (5.57%), 2 were angiomatous (2.22%), and 2 secretory (2.22%) [Figures 1–5]; there were 4 cases of atypical meningiomas (4.44%), 3 were clear cell (3.33%) and 1 case of anaplastic meningioma (1.11%). According to the WHO grading system, 82 were Grade I (91.11%), 7 were Grade II (7.78%), and 1 was Grade III meningioma (1.11%). Only 1 Grade 3 is found in spinal region, 4 cases of Grade 2 are found in spinal region, and 2 cases of Grade 2 in sphenoidal region [Table 1]. Out of 82 cases of Grade 1, 59 cases (71.95%) were in female, 23 (28.05%) in male, and in Grade 2, out of 7 cases, 4 (57.14%) were in male and 3 (42.86%) in female, 1 case of Grade 3 was in male. Hence, Grade 1 meningioma is more common in female and Grade 2 is more common in male. There was only 1 case of Grade 3, anaplastic meningioma which was in male. The mean age of patients in Grade 1 was 42.16 ± 15.13 years, whereas it was 34.21 ± 24.04 in Grade 2. No significant difference was seen in relation to the age in patients with different grades of tumor (P > 0.172). Immunohistochemical staining was done in all cases [Table 2]. Among all tumors (90 cases), nuclear immunostaining for PR was positive in 79 (87.78%) cases [Figure 6] and negative for 11 (12.22%) cases. PR was positive in 79/82 (96.34%) of Grade I, none (0%) of Grade II tumors. The difference between groups was statistically significant (Fisher's exact test two-tailed P < 0.001) [Table 3]. Higher PR positivity is seen in Grade 1 tumors and all Grade 2 tumors were PR negative. Out of 62 female patients, PR was positive in 59 (95.16%) cases and negative in 3 (4.84%) cases. Out of 28 male patients, 20 (71.43%) were positive for PR, 8 (28.57%) were negative. Nuclear immunostaining for ER was positive in 11 (12.22%) out of total 90 cases, 3/82 (3.66%) of Grade 1 and all (100%) cases of Grade 2 tumors [Figure 7]. The difference between groups was statistically significant (Fisher's exact test two-tailed P < 0.001) [Table 4]. All Grade 2 tumors were positive for ER. Due to small number of these samples, they were not significant. Mean Ki-67 labeling index (LI) was 2.57 ± 1.674 among Grade I tumors, 7.11 ± 1.084 in Grade II meningiomas [Figure 8]. Comparison of Ki-67 between two grades was significant (Mann–Whitney U-test P < 0.001). Ki-67 LI was low in Grade 1 meningiomas and high in Grade 2 meningiomas [Table 5].
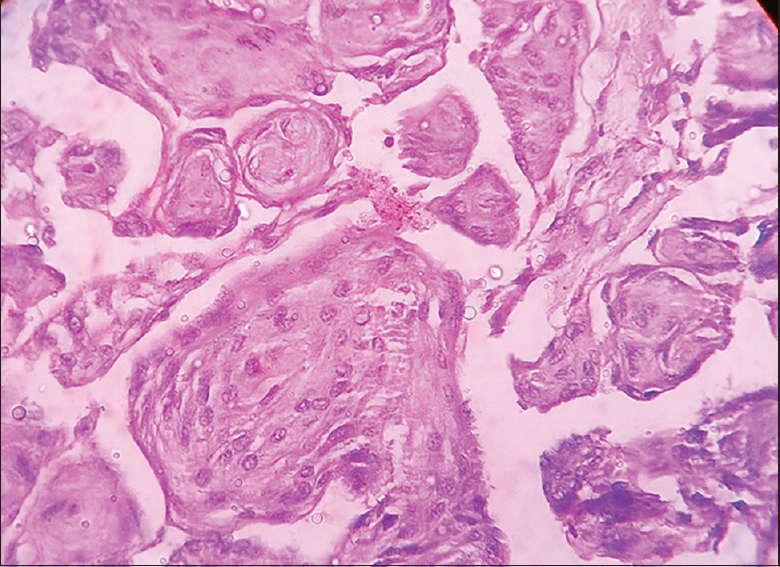
- Photomicrograph showing whorls in meningioma (×400) Grade 1
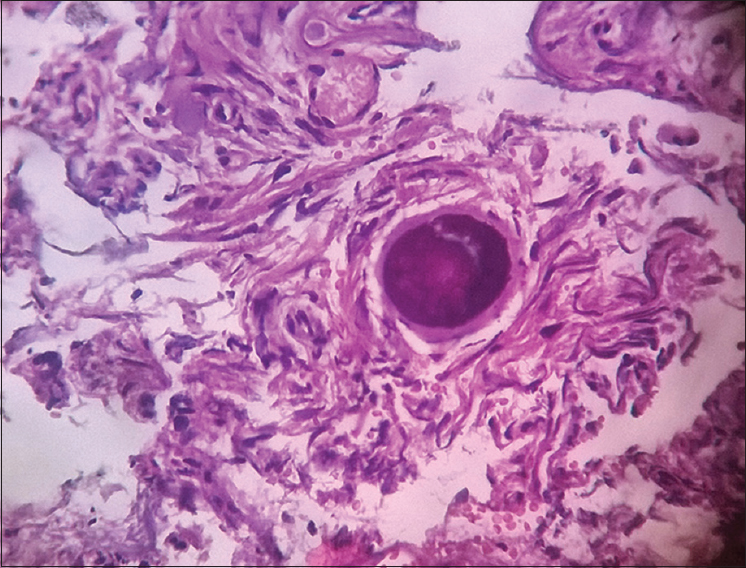
- Photomicrograph showing psammomatous meningioma (×400) Grade 1
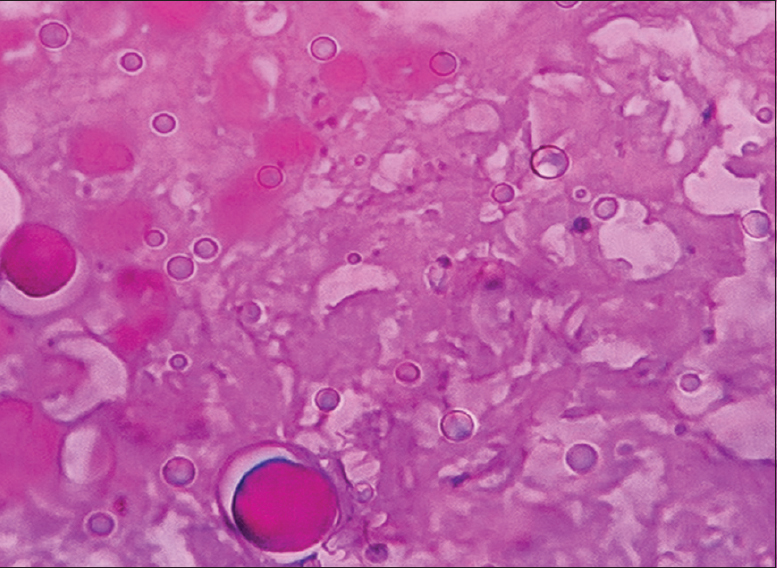
- Photomicrograph showing secretary meningioma (×400) Grade 1
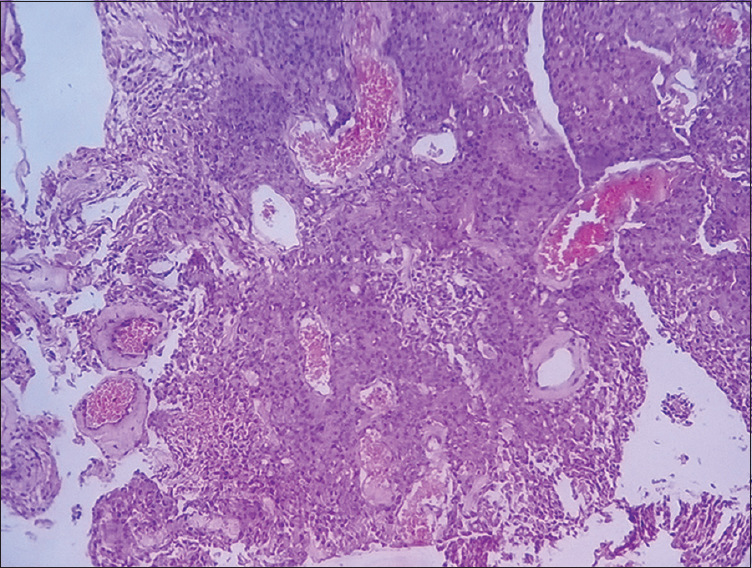
- Photomicrograph showing angiomatous meningioma (×100) Grade 1
- Photomicrograph showing fibroblastic meningioma (×100) Grade 1

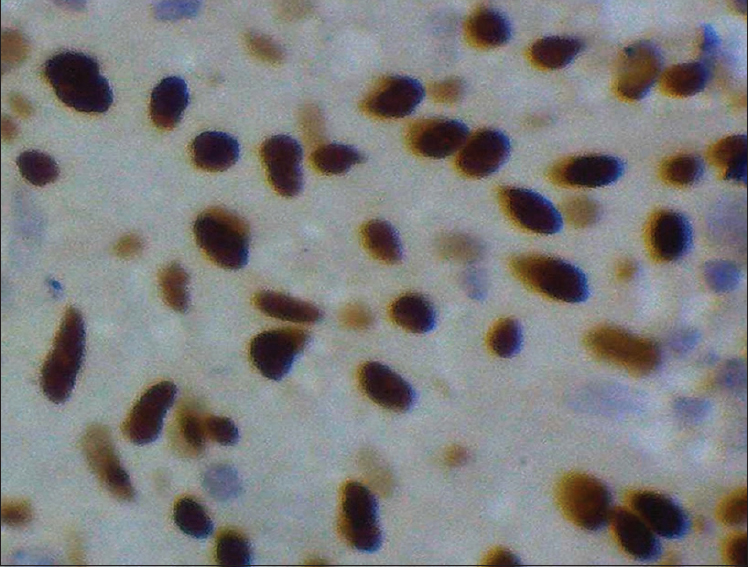
- Immunohistochemical stain showing progesterone receptor positive stain (×400) Grade 1
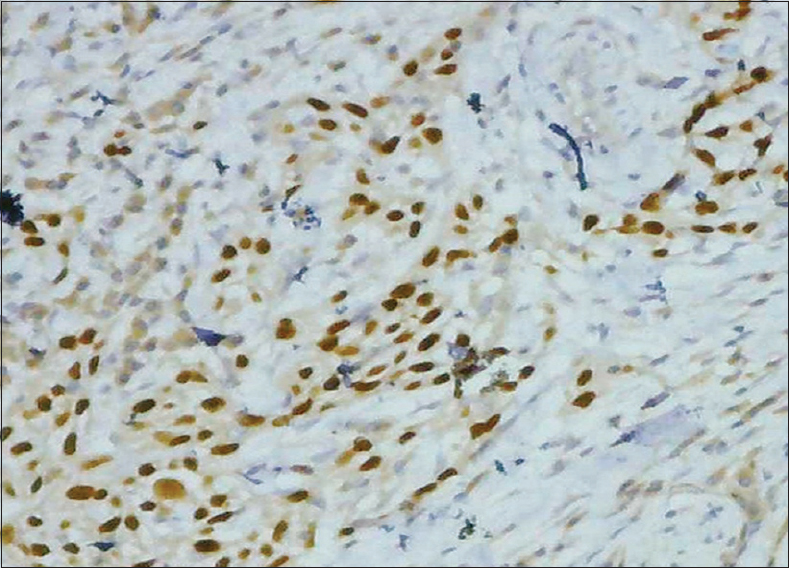
- Immunohistochemical stain showing estrogen receptor positive stain (×100) Grade 2
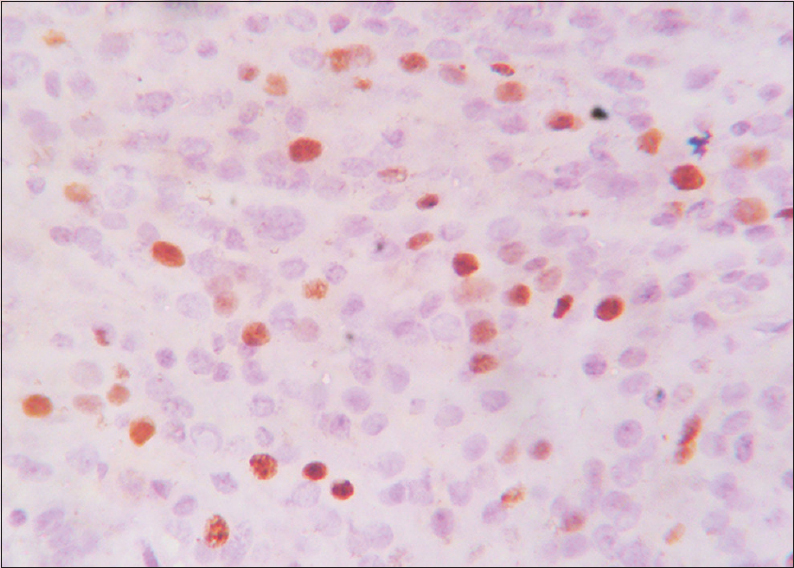
- Immunohistochemical stain showing Ki-67 positivity (×400) Grade 2

Discussion
Many studies have been done till now to correlate the role of ER, PR, and Ki-67 in case of meningioma and also to assess their importance as prognostic markers in predicting the behavior of meningioma, but different studies have given varied conclusion regarding the correlation of ER, PR, and Ki-67 expression with the biological behavior of meningiomas. Out of the 90 evaluated cases, 35 were meningothelial (38.89%), 18 fibroblastic (20%), 20 transitional (22.22), 5 psammomatous (5.57%), 2 were angiomatous (2.22%), and 2 secretory (2.22%); there were 4 cases of atypical meningiomas (4.44%), 3 were clear cell (3.33%) and 1 case of anaplastic meningioma (1.11%). Our result is same as that of Gursan et al.[9] who also showed higher percentage of meningothelial, transitional subtype, and lower percentage of fibrous meningiomas. Gursan et al. found 50% meningothelial, 16.3% fibrous, 29.2% transitional, 1.8% atypical, and 2.7% anaplastic meningiomas in their study. Roser et al.'s study showed that there was significantly lower PR expression in WHO Grade II (atypical) and III (anaplastic) meningiomas compared with WHO Grade I meningiomas (P < 0.0001).[8] In our study, we found same result as PR positivity is seen in 96.34% of Grade 1 tumors and none of Grade 2 tumors. Hilbig and Barbosa-Coutinho's study differed from our study; it showed among Grade 1 tumors, 58.3% were positive for PR while 48.2% were positive among Grade 2.[10] In this study, they showed all tumors analyzed for ER were negative, both Grade 1 and Grade 2 meningiomas, different from our study which shows ER was positive in 3.66% of Grade 1 and all cases of Grade 2 tumors. Shayanfar et al.'s study showed nuclear immunostaining for PR was positive in 96.8% of Grade I and it was same as our study as we found PR positivity in 96.34% of Grade 1 cases, but it showed PR positivity in 20% of Grade 2 which differs from our study which showed all Grade 2 tumors were PR negative and this study showed none (0%) of Grade 3 tumors were positive for PR as in our study. The difference between groups was significant as in our case (P < 0.001).[11] Fewings et al.[12] showed higher prevalence of malignant tumors in males as in our study but it showed no significant relationship between positive PRs and sex; but, in our case, we found PR positivity more in female cases. In Wahab et al.'s study, the PRs were positive in 91% of males, and 81% of females; but, in our case, PR was positive in 95.16% of female and 71.43% of male. No relationship was seen between age and tumor malignancy grade, but malignant tumors were more frequent in males as in our study. In this study, positive PRs are significantly more in benign tumors than malignant ones and PRs were associated with a better prognosis similar to other studies.[13] The reason for the relationship between PR and grading of meningioma is not clearly known yet, but it could probably be due to the higher incidence of mitosis in tumor cells in the presence of low number of PRs and cellular turnover[12] and also increase in angiogenesis occurs in the absence of PRs.[14] It was also reported that positive PRs were associated with less recurrence of the tumor.[14] Walter et al.'s study showed that administration of preoperative medroxyprogesterone in patients with positive PRs, tumors would result into a better clinical response compared to patients having tumors without any PR.[15] Shayanfar et al.'s study showed mean Ki-67 LI was 2.98 ± 2.27 among Grade 1 tumors, 9.30 ± 5.79 in Grade 2 which was also similar to our study where we found mean Ki-67 LI was 2.57 ± 1.674 among Grade 1 tumors, 7.11 ± 1.084 in Grade 2 meningiomas. Differences between two grades were significant (P < 0.001) like our study[11] Roser et al. showed the presence of significantly positive PR in benign meningiomas compared with WHO Grade 2 or 3 tumors. They also found that PR status affected survival only in combination with the proliferation marker Ki-67.[8] Wolfsberger et al. also found that PR positivity was mostly seen in patients under the age of 50 years with WHO Grade 1 meningiomas of the meningothelial subtype and low cell proliferation index.[16] No correlation was found between ER and PR status as found in case of breast and uterus. Benign tumors were associated with low PI, high PR positivity, and ER negativity. Meningiomas with higher proliferation index and negative PR were very likely to be Grade 2 or Grade 3. PR positivity was mostly seen in female patients. Grade 2 tumors were most common in male. There were no correlation between age and grading of tumors. As presence of PRs is more common in women, prevention of pregnancy is advised in women suffering from this tumor or it should be treated before any pregnancy. In women having meningioma, administration of contraceptive pills containing progesterone is not recommended.[15]
Conclusion
We reviewed the 90 cases of meningiomas to examine the presence of ER, PR, and Ki-67 by immunohistochemical methods and the possible relationships between their immunostaining status and other tumor parameters. The results are summarized as follows: (a) Benign tumors were associated with low PI, high PR positivity, and ER negativity. (b) Meningiomas with higher proliferation index and negative PR were very likely to be Grade 2 or Grade 3. (c) PR positivity was mostly seen in female patients. (d) Grade 2 tumors were most common in male. (e) There were no correlation between age and grading of tumors. Surgery is the treatment of choice; but in cases such as aging, medical problems, inaccessibility to tumor, incomplete removal, and recurrence or in malignant type, surgery is not possible and will not be the only sufficient choice. If these cases are presented with positive PRs, in addition to radiotherapy, hormonal manipulation can be used.
Follow-up study
Meningiomas are known for frequent recurrence, even after complete resection. Prediction of recurrence cannot be done by histomorphological features alone. Hormonal receptor status and cell proliferation indices are used as a guide in grading of meningioma, and therefore, it helps in predicting the recurrence potential of tumor. Meningioma with negative PR and higher PI is likely to be atypical (Grade 2) or malignant (Grade 3) and potentially considered to be recurrent. As it is a single institute-based study and the study period being very short, the number of case is small. Hence, it is not sufficient to give generalized result to comment on whole population. Survival analysis of meningioma patients cannot be done as follow-up was not possible for the short time limit. Larger population-based trial is required to delineate prevalence, clinicopathological features, and prognosis of the patient, and longer follow-up is required to study the recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The accuracy of meningioma grading: A 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141-9.
- [Google Scholar]
- Epidemiology of primary intracranial tumours in NW Italy, a population based study: Stable incidence in the last two decades. J Neurol. 2002;249:281-4.
- [Google Scholar]
- Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968-1997. Int J Cancer. 2005;117:996-1001.
- [Google Scholar]
- Atypical and anaplastic meningiomas: Radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233-42.
- [Google Scholar]
- Meningiomas. In: Kleihues P, Cavenee WK, eds. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000. p. :134-41.
- [Google Scholar]
- The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol. 2004;57:1033-7.
- [Google Scholar]
- Immunohistochemical detection of progesterone receptors and the correlation with Ki-67 labeling indices in paraffin-embedded sections of meningiomas. Int J Neurosci. 2002;112:463-70.
- [Google Scholar]
- Meningiomas and hormonal receptors. Immunohistochemical study in typical and non-typical tumors. Arq Neuropsiquiatr. 1998;56:193-9.
- [Google Scholar]
- Expression of progestrone receptor and proliferative marker ki 67 in various grades of meningioma. Acta Med Iran. 2010;48:142-7.
- [Google Scholar]
- Long-term follow up of progesterone receptor status in benign meningioma: A prognostic indicator of recurrence? J Neurosurg. 2000;92:401-5.
- [Google Scholar]
- Progesterone receptor, bc1-2 and bax expression in meningiomas. J Neurooncol. 2002;56:35-41.
- [Google Scholar]
- The role of progesterone in endometrial angiogenesis in pregnant and ovariectomised mice. Reproduction. 2005;129:765-77.
- [Google Scholar]
- Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg Rev. 2004;27:238-45.
- [Google Scholar]





