Translate this page into:
Evaluation of new indigenous “point-of-care” ABO and Rh grouping device
Address for correspondence: Dr. Aseem Kumar Tiwari, Department of Transfusion Medicine, Medanta - The Medicity, Sector-38, Gurgaon - 122 001, Haryana, India. E-mail: aseemtwr@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Erycard 2.0 is a “point-of-care” device that is primarily being used for patient blood grouping before transfusion.
MATERIALS AND METHODS:
Erycard 2.0 was compared with conventional slide technology for accuracy and time taken for ABO and Rh forward grouping result with column agglutination technology (CAT) being the gold standard. Erycard 2.0 as a device was also evaluated for its stability under different storage conditions and stability of result till 48 h. In addition, grouping of hemolyzed samples was also tested with Erycard 2.0. Ease of use of Erycard 2.0 was evaluated with a survey among paramedical staff.
RESULTS:
Erycard 2.0 demonstrated 100% concordance with CAT as compared with slide technique (98.9%). Mean time taken per test by Erycard 2.0 and slide technique was 5.13 min and 1.7 min, respectively. After pretesting storage under different temperature and humidity conditions, Erycard 2.0 did not show any deviation from the result. The result did not change even after 48 h of testing and storage under room temperature. 100% concordance was recorded between pre- and post-hemolyzed blood grouping. Ease of use survey revealed that Erycard 2.0 was more acceptable to paramedical staff for its simplicity, objectivity, and performance than conventional slide technique.
CONCLUSION:
Erycard 2.0 can be used as “point-of-care” device for blood donor screening for ABO and Rh blood group and can possibly replace conventional slide technique.
Keywords
ABO grouping
Column Agglutination Technology
donor screening
ease of use
Erycard
lateral flow
point of care
stability
Introduction
The basic serological technique in any blood transfusion service is ABO and Rh grouping, the principle of which is based on specific agglutination reaction between antigen on red cells and antibodies in the serum. ABO blood grouping is done in two steps; first is the red cell typing or forward grouping and the second step is the serum or reverse grouping. However, Rh grouping is done in a single step, that is, forward grouping.
There is a wide range of various analytical tests available for ABO and Rh blood group typing. Some are age old classical ones such as tube or slide tests, whereas some are relatively modern day methods such as solid-phase red-cell adhesion and column agglutination technology (CAT).
Grouping by slide method has a lot of limitations. It has been proved that slide grouping should always be supplemented with a more robust grouping technique comprising both cell and serum grouping.[1] Some of the limitations of slide method include drying up of reaction mixture, difficulty in interpreting weaker reactions, mixing up of reaction mixtures, misinterpretation due to inadequate mixing of RBC and antisera, no reproducibility, and many others.[2] Despite being less sensitive, it is still used as preliminary and usually point-of-care (POC) technique because of its simplicity and ease of use, especially in resource-constrained settings.[3] Recently, a new indigenous POC device Erycard 2.0 has been introduced for determining ABO and Rh blood groups which is based on the principle of lateral flow guided by capillary action. This is similar to the slide grouping in terms of simplicity, ease of use, no requirement of equipment or extensive training, and also overcomes several limitations of slide grouping.
This study was undertaken with an aim to evaluate and compare the accuracy of Erycard 2.0 against conventional slide technique with CAT as the gold standard. In addition, ease of use, grouping of hemolyzed samples, stability of the device, and stability of the results given by Erycard were also tested.
Materials and Methods
Settings and design
This was a prospective, analytical study performed at a tertiary health-care-based blood bank on blood donors from July to August 2016. The blood bank collects around 25,000 whole blood units annually.
Erycard™ 2.0 blood grouping test
Erycard 2.0 blood grouping card for ABO and Rh(D) forward grouping with autocontrol is based on the principle of lateral flow. It is a POC device manufactured by Tulip Diagnostics Ltd., Goa, India. Using the fixed volume micropipette provided, 5 μl of test participant's whole blood sample was added to each of the 4 wells, ensuring that only the blood drop was in contact with the reagent. After 1 min, two drops of buffer were added to each well. After waiting for 3 min, the results were interpreted. The autocontrol should always show a colorless patch for valid interpretation.
Conventional slide grouping
On a clean slide, one drop of Anti-A, Anti-B, and Anti-D were taken, and three drops of blood were added to the drop of antisera. Each solution was mixed carefully with a separate applicator stick. The slide was rocked back and forth slowly for around 1 min and then agglutination was recorded.
Automated Column Agglutination Technology
CAT was considered as the gold standard method for blood grouping. Blood group for all donors was performed by automated CAT-based equipment (AutoVue Innova, Ortho Clinical Diagnostics, UK). This technology is objective, sensitive, straightforward, and relatively easy to operate.
Comparison of blood grouping between slide and Erycard 2.0
For comparison of accuracy of blood grouping between slide and Erycard 2.0
This comparison was performed on 550 consecutive blood donors. Using a single fingerprick, capillary blood sample was taken for grouping by slide and Erycard. Grouping by CAT in AutoVue was done from the venous sample obtained from the donor at the time of donation. All samples whose results were concordant on slide, Erycard and AutoVue grouping were considered correct. For samples, where there was discordance between Erycard 2.0 and slide; AutoVue result was considered final.
For comparison of time span for slide grouping and grouping by Erycard 2.0
This comparison was performed on additional consecutive fifty blood donors. Time taken to perform grouping by slide and that by Erycard was measured using a stopwatch starting with finger prick and ending at interpretation of result.
Other evaluations of Erycard 2.0
Assessing the effect of temperature and humidity on the devices
To study the effect of storage, temperature, and humidity conditions, 24 devices each were kept in four different environmental setups for 30 days and then tested simultaneously. The four setups included high temperature with high humidity, high temperature with low humidity, low temperature with low humidity, and low temperature with high humidity. A control group of 24 devices was also kept at the optimum temperature (2°C–30°C), as described in the manufacturer's instructions. The humidity for control was maintained between 30% and 35%.
In all the settings, the container and thermohygrometer were checked every day for 30 days. The cards were taken out on the 31st day. Using 24 known donor blood samples (containing both Rh D positive as well as Rh D negative samples), blood grouping was performed on devices kept in setting 1,2,3,4 and control simultaneously. The results were recorded and compared.
Setting 1: High temperature with high humidity.
A dry incubator was set at 45°C. Open containers filled with water were placed on all shelves. A thermohygrometer was placed inside the incubator to record the temperature and humidity. The devices were placed in the incubator. The humidity was maintained between 70% and 75%.
Setting 2: Low temperature with high humidity.
Twenty-four devices were placed in a container with open surface in the cold room. A thermohygrometer was placed inside the container to record the temperature and humidity. The temperature ranged between 4°C and 6°C, and humidity was maintained between 80% and 85%.
Setting 3: High temperature with low humidity.
Twenty-four devices were placed in a container with the thermohygrometer, and the temperature was set at 45°C. The humidity was maintained between 10% and 15%.
Setting 4: Low temperature with low humidity.
An airtight container was taken and kept inside the incubator, when warm it was taken out and silica gel was placed inside it along with 24 devices and the thermohygrometer. The container was closed immediately and was wrapped with cellophane tightly. The container was transparent, and the thermohygrometer was placed in such a position that it could be read at any time. This setup was placed in the cold room at 4°C–6°C, and the humidity was maintained between 30% and 35%.
Assessing stability of results in Erycard 2.0
To test the stability of the results obtained by Erycard 2.0, blood grouping of unknown fifty donor samples was performed. The initial results were recorded, and this was considered as 0 h. The devices were left at room temperature and interpreted after every 6 h. The interpretations were recorded at the end of every 6 h, and this was done till 48 h after which the devices were discarded.
Assessing the effect of hemolysis on the accuracy of blood grouping by Erycard 2.0
To test the effect of hemolyzed samples on the accuracy of the device, blood grouping of known samples (5 each of A positive, A negative, B positive, B negative, AB positive, AB negative, O positive, and O negative) was performed by personnel 1. After recording the blood groups, samples were centrifuged, plasma was removed, and distilled water was added to the red cells and centrifuged again. After centrifugation, the supernatant was checked for hemolysis, and hemolyzed samples were mixed thoroughly before performing blood grouping. Blood grouping using Erycard 2.0 was performed on these hemolyzed samples and recorded by personnel 2.
Survey for “ease of use” of Erycard 2.0
A survey was conducted for 28 paramedical staff working in blood bank including nursing staff and laboratory technicians to assess the acceptance of Erycard 2.0 over slide method. The questionnaire had four questions and was a 4-point Likert scale. All participants were explained the technique and were asked to perform the same on unknown samples. After performing the test, they were asked to fill up the questionnaire individually.
Statistical analysis
Differences in the discordant grouping results between conventional slide grouping, and blood grouping by Erycard were analyzed and sensitivity and specificity for the new method were calculated.
Ethics Committee approval
The 20 μl whole blood sample that was required for Erycard 2.0 blood group testing was obtained from the same finger prick as the sample for slide grouping; additional prick was not done. Since no donor discomfort was involved in acquiring the sample, hence the institution waivered off the consent and ethical approval.
Results
Comparison of blood grouping between conventional slide method and Erycard 2.0 was performed on two parameters; accuracy of result on 550 blood donors and time span to result on additional 50 blood donors. Evaluation of Erycard 2.0 was performed on four parameters; effect of temperature and humidity on 96 devices, stability of results was studied on 50 devices, effect of hemolysis on accuracy of blood grouping on 40 devices, and a survey for ease of use was also conducted.
For comparison of blood grouping between slide and Erycard 2.0
Comparison of accuracy of blood grouping between slide and Erycard 2.0
A total of 550 healthy, volunteer blood donors were tested by both conventional slide grouping and by Erycard 2.0 and compared with CAT (gold standard).
Concordant results were obtained in 544/550 (98.9%) samples. Out of the six discrepancies that occurred, none were given by Erycard. The positive predictive value of Erycard was 100% and sensitivity was 100% [Table 1]. Out of the six discrepancies, one was an ABO discrepancy, whereas five were Rh discrepancies [Table 2].

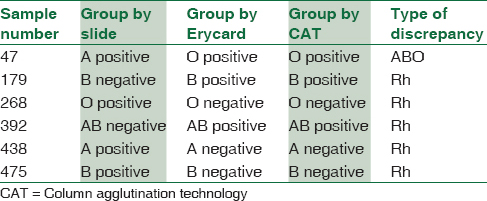
Comparison of time span for slide grouping and grouping by Erycard 2.0
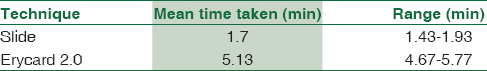
Time taken to perform blood grouping on Erycard 2.0 and slide method was recorded using a stopwatch on fifty samples. The mean of the time taken was calculated [Table 3].
Evaluation of Erycard 2.0
Assessing the effect of temperature and humidity on the devices
The devices stored at four different environmental conditions for 30 days each showed that there is no effect of temperature and humidity variations on the accuracy of blood grouping by Erycard.
Assessing the stability of results obtained by Erycard 2.0
All fifty devices showed no deviation from the initially observed result at 6 h intervals till 48 h.
Assessing the effect of hemolysis on the accuracy of blood grouping by Erycard 2.0
All 40 tests showed the same blood group before and after hemolysis.
Survey for ease of use of the new device
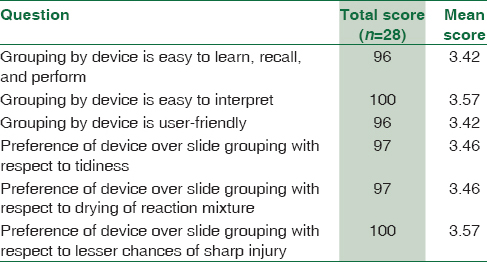
Twenty-eight paramedical staff of blood bank participated in the study. On the basis of the responses obtained from the questionnaires, the mean score for each question was calculated [Table 4].
Discussion
Even today, several blood banks in India use slide grouping as a preliminary method for blood grouping. At present, several POC devices are available for forward grouping which are being used for bedside grouping of patients, but these devices can be used in donor screening as well. POC testing for ABO and Rh blood group finds use in the primary labeling of blood bags at the time of donation which is necessary for maintaining the inventory and also as a check for the final labeling of blood bags. Furthermore, initial blood grouping is important when looking for same blood group donors to perform plateletpheresis and for buffy coat pooling.
The present study was conducted to evaluate Erycard 2.0 as a blood grouping test for blood donor screening. The results from this study demonstrate that ABO and Rh determination with a simple POC device is easy and accurate. Although slide grouping is still used at many centers, it has a lot of drawbacks and Erycard 2.0 can replace grouping by slide in places where grouping might help decrease the errors leading to mismatched blood transfusion.
In the present study, the device demonstrated 100% concordance with CAT, the gold standard. In 2015, El Kenz and Corazzatested a POC ABO agglutination test device and observed that there was 100% concordance between the POC testing device and their laboratory instruments.[4]
However, Dhruva et al.[3] conducted a study in 2015 on the accuracy of Erycard 2.0, after which they concluded 97.6% concordance between results obtained by Erycard and that by their gold standard (conventional tube technique).[5] They found 7/300 discrepancies in patient samples tested and the discrepancies were due to low hematocrit (<15%), autoclumping, anti-A1 antibody, and hemolyzed sample. However, since the present study was conducted on donor sample obtained from a fingerprick, the above-mentioned causes of discrepancy were not pertinent to the present study.
The device is designed as a POC test to be used with freshly obtained whole blood. The evaluation of Erycard 2.0 was done using whole blood from a fingerprick. This was an advantage over the study conducted by Thomas Herold et al., who performed their testing on previously collected stored samples.[6] Hemolysis and sample degradation could result from handling variations and prolonged storage and thus cause deviation in results.
In the present study, there was significant difference in the average time taken for blood grouping by Erycard 2.0 and by the slide. Although the time taken by Erycard was more, the method was less messy and more objective as compared to slide method of blood grouping.
In 2009, Bienek et al. conducted a study to test the stability of user-friendly blood typing kits stored under typical military field conditions[7] Eldon Home Kit 2511 (Eldon Biologicals A/S, Denmark) and ABO-Rh Combination Blood Typing Experiment Kit (Lab Aids, Inc., NY, USA) were used. No differences were found between results from kits stored under manipulated storage conditions and those stored at optimum storage conditions. These results were similar to the results obtained in the present study, which indicate that during transportation, even if the devices are exposed to unfavorable temperature and humidity conditions, the accuracy of blood grouping obtained by Erycard 2.0 is not affected.
In the present study, no deviations were observed in all the tested devices from the initial result, till 48 h after testing. As per the manufacturer's instructions, for stable results, the devices must be stored in a sealed cover without contamination in a cool, dry place, and avoid exposure to direct sunlight and heat. The tested devices in the present study were left open at room temperature which is maintained between 20°C and 24°C normally. This observation is important when results need to be stored to solve blood grouping discrepancies while labeling of blood bags and also when donors come to blood banks with doubts about their blood group.
Hemolyzed samples may produce erroneous results of many laboratory tests including blood group testing. Supernatant hemoglobin can produce discrepancies between forward and reverse group. Hence, determining the exact blood group of hemolyzed samples is difficult. In the present study, it was observed that Erycard 2.0 determines all blood groups (A positive, A negative, B positive, B negative, AB positive, AB negative, O positive, and O negative) of hemolyzed samples correctly. This observation is extremely important since with even the most sensitive techniques, sometimes transfusion services are unable to comment on the blood group of hemolyzed samples.
The results obtained from the survey conducted for the paramedical staff suggested that staff agreed that the new device is easy to learn, recall, perform, interpret, and is a user-friendly. The staff preferred Erycard 2.0 over slide grouping due to its tidiness, no drying of the reaction mixture, and less chances of sharp injury.
Conclusion
Erycard 2.0 is easy to use and interpret and even with minimal training blood bank staff can perform blood grouping easily. The device can become a useful tool for determining blood group of hemolyzed samples. Overall accuracy of the device is better than slide technique and hence can be used as a method of preliminary blood group testing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge Tulip Diagnostics Private Limited, Goa, India, for providing devices free of cost.
References
- Guidance Manual on “ABO and Rh Blood Grouping”. Document ID No. NIB/BRL/GM/02. National Institute of Biologicals (Ministry of Health & Family Welfare) Government of India 2013:10-2.
- [Google Scholar]
- Blood group typing: From classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors. 2015;16:51.
- [Google Scholar]
- Recent and future trends in blood group typing. Anal Bioanal Chem. 2009;393:1443-51.
- [Google Scholar]
- Automated point-of-care testing for ABO agglutination test: Proof of concept and validation. Vox Sang. 2015;109:79-85.
- [Google Scholar]
- Comparison of conventional Tube agglutination method versus Erycard 2.0 for the ABO blood grouping system – A pilot study. Int J Res Med. 2015;4:59-61.
- [Google Scholar]
- Determining the accuracy of a rapid point-of-care test for determining Rh(D) phenotype. Acad Emerg Med. 2005;12:474-6.
- [Google Scholar]
- Stability of user-friendly blood typing kits stored under typical military field conditions. Mil Med. 2009;174:1075-80.
- [Google Scholar]





