Translate this page into:
Volume, conductance, and scatter parameters of neoplastic and nonneoplastic lymphocytes using Coulter LH780
Address for correspondence: Dr. Chidambharam Choccalingam, Department of Pathology, Chettinad Hospital and Research Institute, 25, 6th Main Road, Raja Annamalipuram, Chennai - 600 028, Tamil Nadu, India. E-mail: chidambharam@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
PURPOSE:
Automated hematology analyzers yield a complete hematological profile including a complete blood count and a differential white blood cell count. The differential count is based on analyses of three parameters, namely, volume, conductance, and scatter (VCS). We aimed to evaluate the VCS parameters, histograms, and scatterplots of neoplastic and nonneoplastic lymphocytes.
MATERIAL AND METHODS:
Patients were grouped into four categories, namely, acute lymphoblastic leukemia (ALL), chronic systemic disorders, chronic lymphocytic leukemia (CLL), and acute viral disease. Lymphocytes from all four groups were compared with lymphocytes from normal participants.
RESULTS AND CONCLUSIONS:
The histogram for acute viral disease showed a trough at T1, which was slightly obliterated, and the F1 curve mildly extended to the right. The T1 for ALL was replaced with a peak at >40% of the preset limit. The F1 peak was shifted to left for CLL. The scatterplot for viral disease showed lymphocytes extending to the variant lymphocyte window. The lymphocytes of ALL extended to the blast window, with both increase in volume and mild increase in scatter. The lymphocytes in CLL were smaller and located below the normal lymphocyte region. Mean lymphocyte volume was significantly increased in ALL and was significantly decreased in CLL. Mean lymphocyte conductance was significantly increased in CLL and significantly decreased in both acute viral disease and ALL. Mean lymphocyte scatter was significantly decreased in acute viral disease and significantly increased in ALL.
Keywords
Histogram
neoplastic lymphocytes
nonneoplastic lymphocytes
scattergram
volume
conductance
and scatter
Introduction
Automated hematology analyzers can provide a complete hematological profile that includes a complete blood count and a differential white blood cell (WBC) count.[1] For example, the Coulter LH780 is an automated instrument that can differentiate WBC based on data from three parameters: volume (V), conductance (C), and scatter (S). This approach is relatively rapid and simple although peripheral blood smears remain the gold standard for lymphocyte morphology analysis.[2] Nevertheless, the VCS parameters, histograms, and differential plots can provide information regarding the nature of neoplastic and nonneoplastic lymphocytes.[23] Several studies have established the VCS parameters for atypical lymphoid cells from patients with lymphoproliferative diseases.[4] The present study aimed to evaluate the VCS parameters, histograms, and scatterplots of neoplastic and nonneoplastic lymphocytes.
Materials and Methods
Peripheral blood samples in tripotassium ethylenediaminetetraacetic acid were obtained from healthy donors (29 participants) and patients with acute viral diseases (e.g., dengue fever) (23 participants), chronic systemic disorders (e.g., rheumatoid arthritis and tuberculosis) (14 participants), acute lymphoblastic leukemia (ALL) (2 participants), and chronic lymphocytic leukemia (CLL) (2 participants). The diagnoses of acute viral diseases and chronic systemic disorders were based on serological, immunological, and microbiological data. The diagnoses of ALL and CLL were confirmed using cytological and immunophenotyping data.
A Coulter LH780 (Coulter, India) was used to collect data regarding the total WBC count, lymphocyte count, histogram, differential plot, and VCS positional characteristics for each group of patients. This instrument determines volume using a direct current impedance method, conductivity using high-frequency radio waves that are passed through the nucleus, and light scatter using a laser beam that is scattered by the cytoplasmic granules. Each group's values for mean volume, standard deviation for volume, mean conductance, standard deviation for conductance, mean scatter, and standard deviation for scatter were compared with normal participants value using the Mann-Whitney test. Lymphoblasts were differentiated from myeloblasts using VCS parameters for lymphocytes and neutrophils as well as prototype histograms and differential plots were compared.
Results
The VCS parameters for lymphocytes in each group were compared to data from healthy donors. Prototype histograms [Figure 1] and differential plot from individual patients in each group were compared.
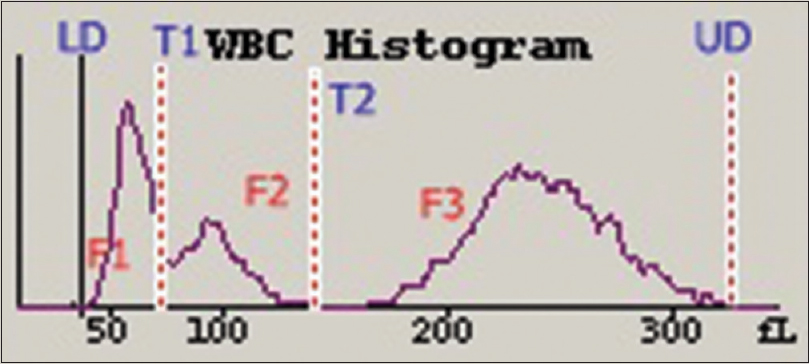
- The white blood cell histogram from a healthy donor reveals a lower discriminator at 30 fl, an imaginary discriminator at 78-114 fl, a second imaginary discriminator (T2) at 150 fl, and an upper discriminator at 300 fl. The F1 peak between lower discriminator and T1 represents lymphocytes. The F2 peak between T1 and T2 represents monocytes, eosinophils, basophils, and immature neutrophils. The F3 peak between T2 and upper discriminator represents neutrophils
Histogram
The trough at T1 from the patients with acute viral disease was obliterated and the F1 curve was slightly shifted to the right [Figure 2a]. The T1 trough for the patients with ALL was replaced with a peak at T1 that was >40% above the preset limit [Figure 2b]. The F1 peak was shifted to left for patients with CLL [Figure 3b].
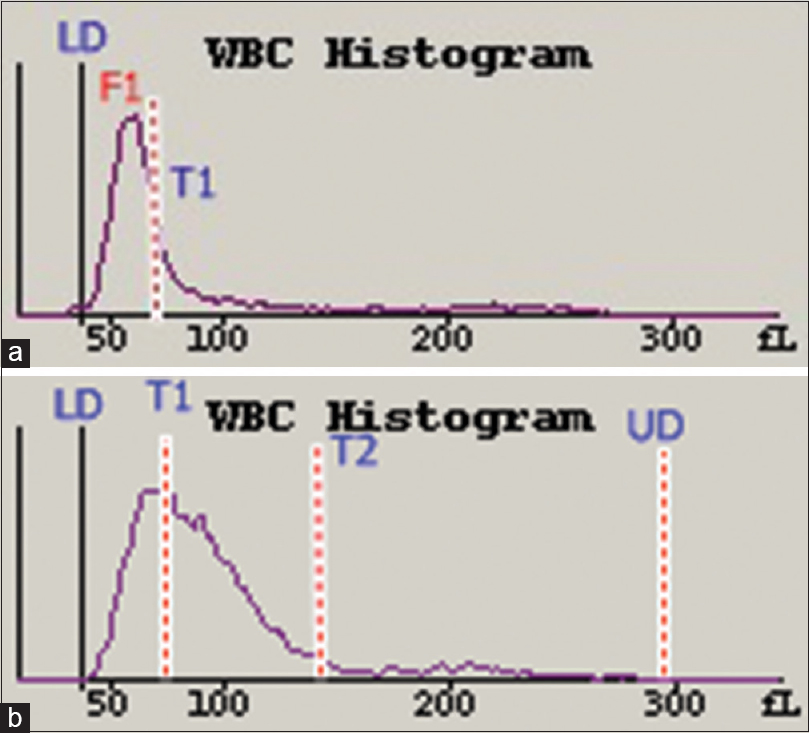
- White blood cell histograms from a patient with acute viral disease (a) and a patient with acute lymphoblastic leukemia (b). The F1 curve in (a) has shifted slightly to the right with obliteration of the T1 trough, <40% preset limit. The T1 trough in (b) is obliterated at >40% of the preset limit
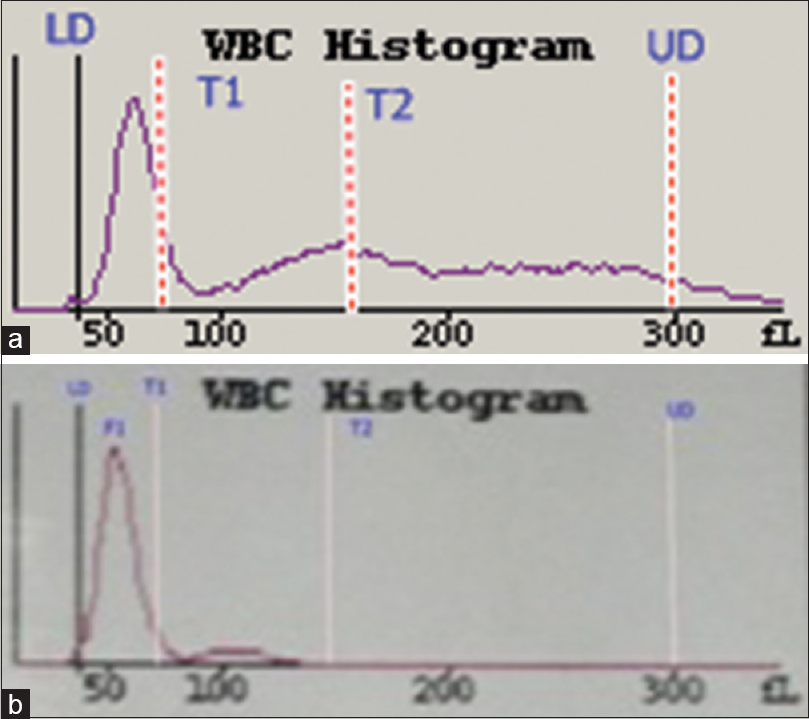
- White blood cell histograms from a patient with a chronic systemic disorder (a) and a patient with chronic lymphocytic leukemia (b). The histogram in (a) is normal while the histogram in (b) shows that the F1 curve has shifted to the left
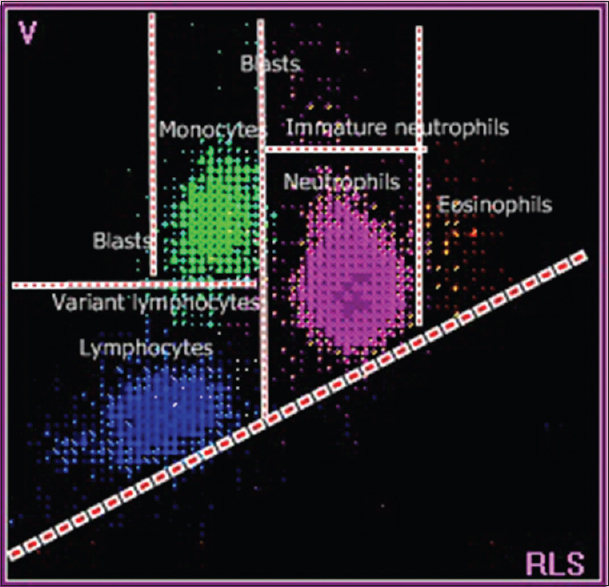
- A scattergram with labeled white blood cell populations from a healthy donor. The blue dots indicate lymphocytes, green dots indicate monocytes, pink dots indicate neutrophils, and orange dots indicate eosinophils
Scattergram
The patient with acute viral disease had lymphocytes that extend into the variant lymphocyte window [Figure 5a]. The patient with ALL had lymphocytes that extended into the blast window, with greater volume and scatter values [Figure 5b]. The patient with CLL had relatively small lymphocytes, and cells were present below the normal lymphocyte window [Figure 6b].
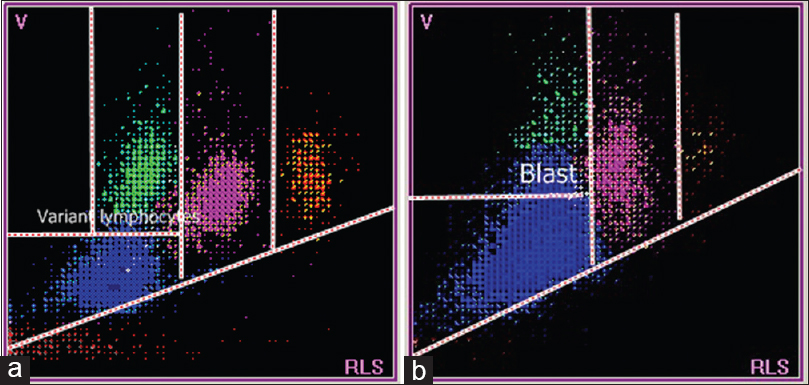
- Scattergrams from a patient with acute viral disease (a) and a patient with acute lymphoblastic leukemia (b). (a) Lymphocytes are plotted in variant lymphocyte window which is slightly above and to the right of normal lymphocyte population. (b) Lymphocytes in the blast window which is well above the normal lymphocyte population
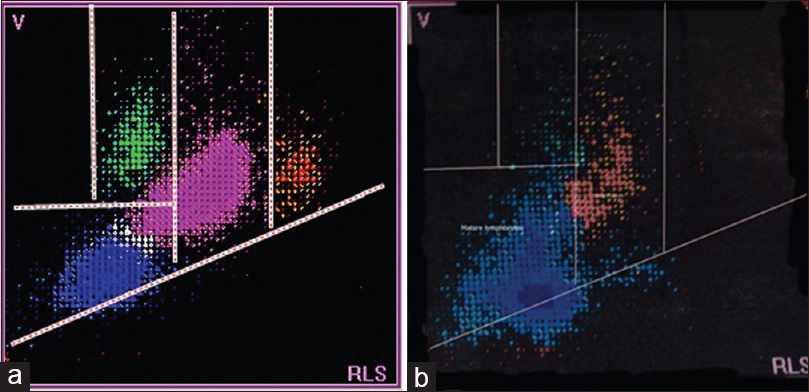
- Scattergrams from a patient with a systemic disorder (a) and a patient with chronic lymphocytic leukemia (b). The scattergram in (a) is normal while the scattergram in (b) shows relatively smaller sized lymphocytes, located below the normal lymphocyte population
Comparing the volume, conductance, and scatter parameters
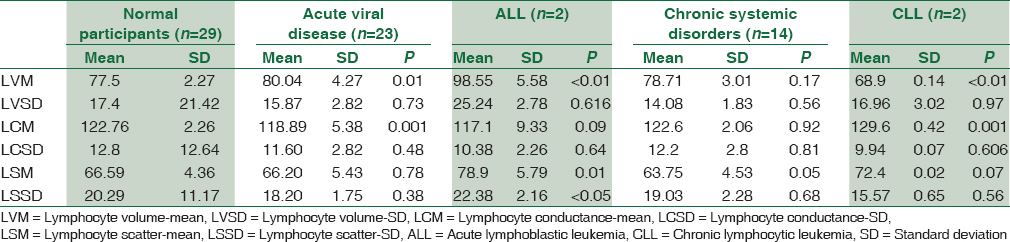
Compared to the healthy donors, the mean lymphocyte volume was significantly increased in patients with ALL and was significantly decreased in patients with CLL. No significant intergroup differences were observed for standard deviation of lymphocyte volume. Patients with CLL had significantly elevated mean lymphocyte conductance although significantly decreased values were observed for patients with acute viral disease and ALL. No significant intergroup differences were observed for standard deviation of lymphocyte conductance. Patients with acute viral disease had significantly decreased mean lymphocyte scatter, although significantly elevated values were observed for patients with ALL. No significant intergroup differences were observed for standard deviation of lymphocyte scatter [Table 1].
Comparing the histograms and scattergrams of patients with acute lymphoblastic leukemia and acute myeloid leukemia
The T1 trough was replaced by a peak, in patients with ALL, whereas the T2 trough was obliterated by a tail whose peak was at about 100 fl, in patients with acute myeloid leukemia (AML) [Figure 7]. The AML scattergram showed abnormal cells above and to the right of the abnormal cells in the ALL scattergram [Figure 8].
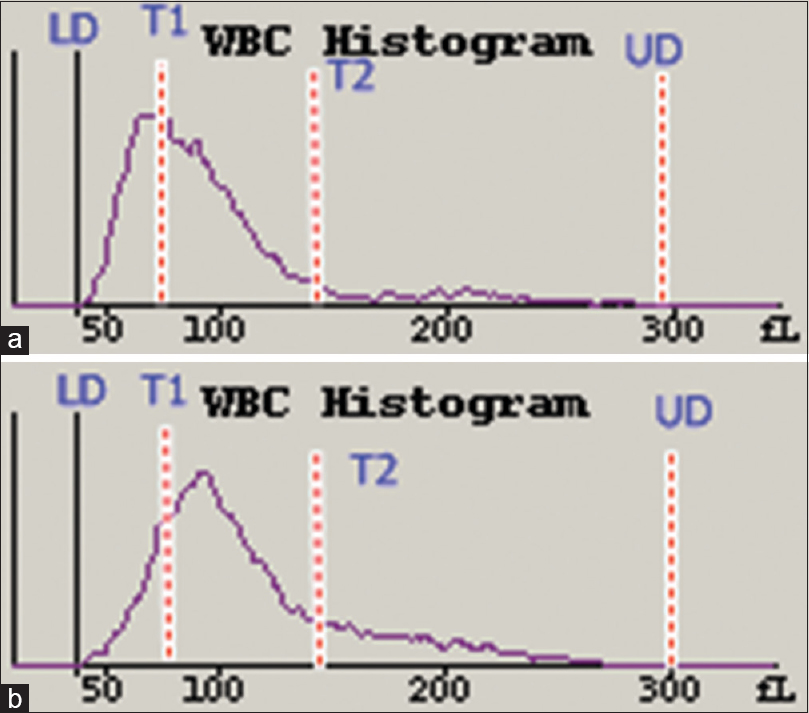
- White blood cell histograms from a patient with acute lymphoblastic leukemia (a) and a patient with acute myeloid leukemia (b). A peak is seen obliterating T1 trough in (a), and a peak is seen at around 100 fl, with mild obliteration of T2 trough in (b)
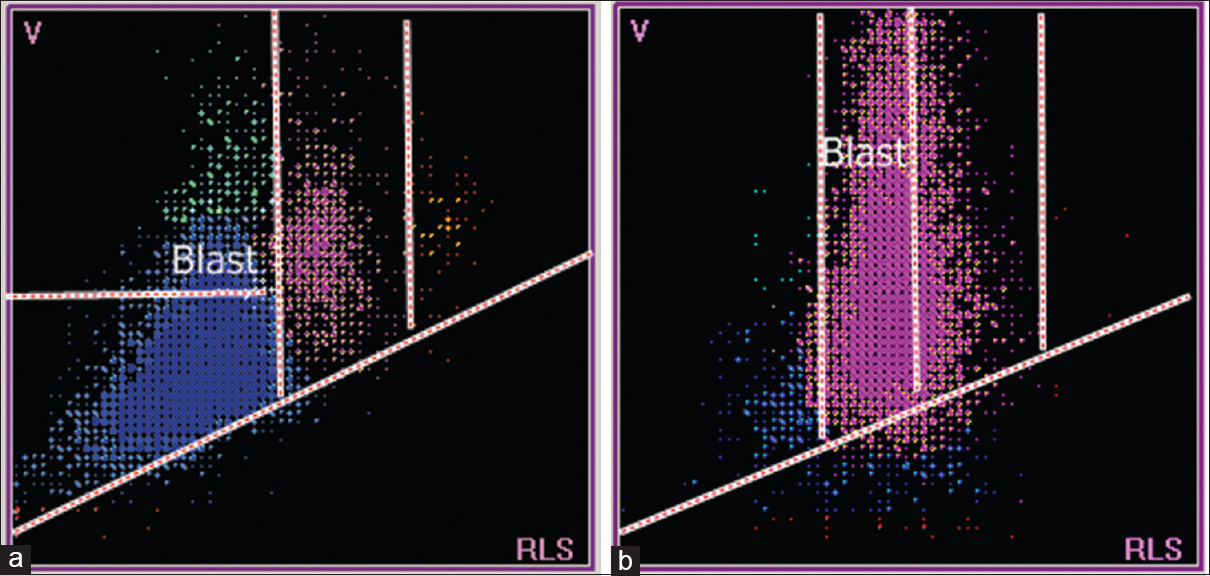
- Scattergrams from a patient with acute lymphoblastic leukemia (a) and a patient with acute myeloid leukemia (b). (a) Lymphocytes in the lymphoblast region which is well above normal lymphocyte population. (b) Neutrophils in the myeloblast region which is well above normal neutrophil population
Discussion
The WBC histogram of healthy donors is contained between the lower discriminator (LD) and upper discriminator, with troughs at T1 (78–114 fl) and T2 (150 fl). The peak between the LD and T1 represents small cells (35–90 fl) that are mainly lymphocytes, and the peak between T1 and T2 represents medium-sized cells (90–160 fl), which include eosinophils, basophils, and monocytes. The peak after T2 represents large cells (160–300 fl) that are mainly neutrophils [Figures 1 and 4].[1] In acute viral disease, the lymphocytes are larger and have less scatter, compared to lymphocytes from healthy donors. These larger lymphocytes obliterate the T1 trough, shift the curve to the right [Figure 2a], and push lymphocytes into the variant lymphocyte window of the scattergram [Figure 5a]. Some lymphocytes are very large and may also be pushed into the blast window.[125] The lymphocytes in ALL cases are also relatively large but have more scattering, compared to the control lymphocytes. These abnormal lymphocytes are reflected in the T1 peak [Figure 2b] and can be identified in the blast window that is above and to the right of the variant window in the scattergram [Figure 5b].[124] In CLL, the lymphocytes are much smaller and have greater conductance, compared to normal lymphocytes. These lymphoid cells create a shift to the left in the histogram [Figure 3b] and are lower than the normal lymphocytes in the scattergram [Figure 6b].[134] There were no significant differences between lymphocytes from patients with chronic systemic disorders and that of the healthy donors [Figure 3a and 6a].
Compared to the abnormal cells in ALL cases, the abnormal cells in AML cases have a larger volume and greater scatter. These characteristics are reflected in the T2 peak and the presence of cells in the blast window, above and to the right of the lymphoblast window [Figures 7 and 8].
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The ABC of CBC: Interpretation of Complete Blood Count and Histograms. (1st). New Delhi: Jaypee Brothers Medical Publishers Ltd; 2013.
- [Google Scholar]
- Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program. 2012;2012:475-84.
- [Google Scholar]
- Cell sizing in chronic lymphoproliferative disorders: An aid to differential diagnosis. J Clin Pathol. 1992;45:875-9.
- [Google Scholar]
- Lymphocyte volume and conductivity indices of the haematology analyser Coulter GEN.S in lymphoproliferative disorders and viral diseases. Clin Lab Haematol. 2006;28:1-8.
- [Google Scholar]
- Evaluation of LH780 VCS parameters and lymph index in identifying dengue fever. Indian J Pathol Oncol. 2015;2:77-81.
- [Google Scholar]





