Translate this page into:
Serum levels of interleukin-6 and tumor necrosis factor-alpha in diagnosis and prognosis of gallbladder cancer: a pilot study
*Corresponding author: Bela Goyal, MD Biochemistry, Department of Biochemistry, All India Institute of Medical Sciences, Rishikesh 249203, Uttarakhand, India. lifeline.bela@gmail.com
How to cite this article: Sharma P, Krishnan MPS, Gupta A, Gupta S, Saxena S, Mirza AA, et al. Serum levels of interleukin-6 and tumor necrosis factor-alpha in diagnosis and prognosis of gallbladder cancer: a pilot study. J Lab Physicians. 2024;16:82-8. doi: 10.1055/s-0043-1772772
Abstract
Objectives:
Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are proin- flammatory cytokines that play a major role in tumorigenesis. These biomarkers are relatively unexplored in gallbladder cancer (GBC) for their diagnostic and prognostic utility.
Materials and Methods:
A total of 40 healthy controls and 40 GBC patients were recruited. Serum IL-6 and TNF-α levels were measured, and their diagnostic utility was analyzed using the receiver operating characteristics (ROC) curve. The relationship between clinicopathological variables and serum tumor markers (CEA, CA125, and CA19-9) in identifying GBC patients was also assessed.
Statistical Analysis:
Statistical analysis was performed using IBM SPSS version 25.0 (IBM Corporation, Armonk, New York, United States).
Results:
Serum IL-6 and TNF-α expression were significantly higher in the GBC group (for both IL-6 and TNF-α, p = 0.0001) than in healthy controls. ROC analysis revealed that the areas under the curve for serum IL-6 and TNF-α were 0.89 and 0.71, respectively. The sensitivity and specificity for serum IL-6 were 82.5 and 97.5%, respectively, at an optimal cutoff value of 10.34 pg/mL; for TNF-α, they were 40.0 and 100%, respectively, at a cutoff value of 0.24 pg/mL. There was also a significant difference in serum IL-6 levels between the resectable and nonresectable GBC groups. Serum IL-6 showed a positive correlation with CA125 (r = 0.34, p < 0.05), while no correlation was observed between serum TNF-α and serum tumor markers (CEA, CA125, and CA19-9).
Conclusion:
Serum IL-6 may serve as a diagnostic marker and a predictor of resect- ability, and it can be used in conjunction with other serum tumor markers in GBC.
Keywords
Gallbladder cancer
interleukin-6
tumor necrosis factor-alpha
INTRODUCTION
Gallbladder cancer (GBC) is the most common lethal malignancy among biliary tract cancers worldwide.[1] In India, GBC is among the top 20 cancers, with a prevalence of 1.82 per 100,000.[2] It is found to be more prevalent in female residents of the Indo-Gangetic belt in Northern India.[3] Usually, GBC has a poor prognosis due to delayed diagnosis because of nonspecific symptoms.[3] To date, there are no definitive tumor markers or screening tests for early diagnosis or determining the prognosis of GBC.
GBC is associated with many ethnic and genetic factors.[4] Chronic inflammation due to gallstones is one of the major risk factors for GBC.[5] The link between inflammation and cancer is well documented. Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are both considered the primary mediators of a network of interactive signals. IL-6 and TNF-α are proinflammatory cytokines involved in the pathogenesis of various cancers, with pleiotropic activities including inflammatory response as well as lymphangiogenesis, tumorigenesis, metastasis, cell proliferation, differentiation, and apoptosis.[6]
IL-6 is secreted by monocytes, Kupffer’s cells, keratinocytes, endothelial cells, and Band T-cells. IL-6 signaling is mediated through the Janus kinases (JAK)/signal transducer and activator of transcription proteins (STAT) pathway and leads to the expression of molecules involved in proliferation, angiogenesis, and metastasis.[6] Increased IL-6 expression has been observed in prostatic, lung, breast, and ovarian cancer.[7–10] One study by Liu et al showed higher circulating levels of IL-6 in GBC, suggesting further exploration of inflammatory pathways in GBC.[11] Similarly, another study by Høgdall et al showed serum IL-6 as an independent prognostic factor in biliary tract cancers, including GBC.[12] TNF-α is also a pleiotropic cytokine that has been associated with cancer progression. TNF-α plays a role in cancer promotion via nuclear factor-κb (NF-κb) signaling.[13] TNF-α has been studied in bile, gallbladder cell lines, and gallbladder tissues.[14,15] Chaturmohta et al studied the messenger ribonucleic acid (mRNA) expression of various cytokines, including IL-6 and TNF-α in GBC.[16] They reported an association of increased expression of TNF-α, IL1-β, IL-8, and VEGF with stages and grades of GBC, whereas IL-6 mRNA failed to show any association.
According to previous studies, IL-6 and TNF-α are the most common inflammatory mediators in the tumor microenvironment.[17-19] There is a paucity of studies on the evaluation of inflammatory cytokines as potential serumbased biomarkers in GBC. However, few scattered reports are available in cases of IL-6,11,12 whereas no such study could be found exploring both IL-6 and TNF-α. The present study aimed to assess the diagnostic efficacy of serum IL-6 and TNF-α in cases of GBC, as well as their correlation with different clinicopathological characteristics and frequently employed serum tumor markers, including CEA, CA125, and CA19-9, which are utilized to evaluate tumor response and prognosis.
MATERIALS AND METHODS
This prospective case-control study was conducted after obtaining appropriate approval from Institutional Ethical Committee (Ref. no. 422/IEC/M. Sc/2020). Informed consent was taken from all the patients. Convenient sampling method was used since this was a time-bound study. Patients ≤18 years, previously treated for GBC, those with metachronous and synchronous malignancies, poor performance status, and those who refused to give consent were excluded. Forty patients aged ≥18 years, with fine needle aspiration cytology (FNAC) or histopathologically biopsy-proven cases of GBC, were included in the present study. Contrast-enhanced computed tomography (CECT) of the abdomen and pelvis was done to determine the disease stage. The staging was done as per the American Joint Committee on Cancer (AJCC) eighth staging system. Resectability was assessed based on the radiological investigation. Criteria for unresectability included the involvement of portal vein, hepatic artery, and direct extension into adjacent organs, and presence of omental or peritoneal deposits and enlarged aortocaval lymph nodes. Forty controls were obtained that were matched for age and sex. Healthy controls were selected from individuals who were ≥18 years with normal liver function tests and no other comorbidities.
Blood Samples and Cytokines Measurement
Five milliliters of venous blood was collected after taking informed consent in a plain vacutainer with clot activators (BD Vacutainer, New Jersey, United States). Serum was separated within 1 hour of blood collection after a centrifugation at 1,500 g for 15 minutes. The serum was stored without preservatives at –80°C and then thawed before testing. Serum IL-6 concentration was determined using commercially available human IL-6 high-sensitivity enzyme-linked immunosorbent assay (ELISA) kit (Weldon Biotech, India). The assay employs the quantitative sandwich immunoassay technique using recombinant human IL-6, with antibodies raised against the recombinant proteins. The IL-6 ELISA assay has a performance sensitivity of 0.81 pg/mL.
Serum TNF-α concentration was determined using the commercially available ELISA US (Ultra-Sensitive) kit supplied by Invitrogen (Waltham, Massachusetts, United States). The TNF-α assay had a performance sensitivity of less than 0.50 pg/mL, and the minimum detectable level was 0.09 pg/mL. Optical density was measured using a microtiter plate reader (BioTek Eon High-Performance Microplate Spectrophotometer, United States) at 450 nm. All standards were run in duplicates, and a standard curve was plotted.
Serum CA19-9, CA125, and CEA levels were investigated by chemiluminescence on ADVIA Centaur XP system (Siemens Healthineers, Erlangen, Germany). The samples were run only after a satisfactory level of performance by two levels of internal quality controls (low and high) as per the manufacturer’s instruction. The cutoff levels taken for CA199, CA125, and CEA were 30.9 IU/mL, 30.2 units/mL, and 5 ng/mL, respectively, as per the specific kits used for analysis.
Statistical Analyses
Baseline characteristics and patient clinical data were expressed in percentages. Serum IL-6, TNF-α, and tumor markers were reported as median and interquartile range. Kolmogorov–Smirnov test showed the nongaussian distribution of the values. Receiver operating characteristics (ROC) curve analysis was performed to determine the clinical utility of serum IL-6 and TNF-α in identifying GBC patients and to determine the sensitivity, specificity, and optimal cutoff value of serum IL-6 and TNF-α in GBC patients. Bivariate Mann–Whitney U test was used to compare serum IL-6 and TNF-α levels in GBC patients and control groups and also in reference to metastasis, gallstones, resectability, lymph node involvement, stages, and liver infiltration. Logistic regression was performed to evaluate the association between clinicopathological characteristics and GBC cases. Spearman’s correlation test was used to find the correlation between serum IL-6 and TNF-α with serum tumor markers. A probability (p) value less than 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS version 25.0 (IBM Corporation, Armonk, New York, United States).
RESULT
A total of 80 samples were enrolled in the study, out of which 40 were GBC patients. The mean age of the GBC patient group was 53.6 ± 12.42 years. In all, 80% of GBC patients were females. Abdominal pain (87.5%) was the most common presenting symptom. Most of the patients were presented at an advanced stage, with 65% in stage IV cancer. In total, 75% of the patients were associated with gallstones, and 80% had liver infiltration. Eighty percent of the patients had an unresectable disease and were subjected to palliative chemotherapy. The healthy control group composed of individuals who were matched in terms of age and sex, exhibited normal liver function test results, and did not present with any notable clinical characteristics. The clinicopathological characteristics of GBC cases are summarized in Table 1.
| Variables | Frequency (N = 40) | Values (%) | |
|---|---|---|---|
| Gender | |||
| Male | 8 | 20.0 | |
| Female | 32 | 80.0 | |
| Personal history | |||
| Smoking | 12 | 30.0 | |
| Alcohol | 5 | 12.5 | |
| Clinical presentation | |||
| Abdominal pain | 35 | 87.5 | |
| Jaundice | 18 | 45.0 | |
| Fever | 18 | 45.0 | |
| Palpable mass | 23 | 57.5 | |
| Gallstones | 30 | 75.0 | |
| Gallbladder mass | 36 | 90.0 | |
| Lymph nodes involvement | |||
| N0 | 7 | 17.5 | |
| N1 | 9 | 22.5 | |
| N2 | 24 | 60.0 | |
| Metastasis | |||
| M0 | 15 | 37.5 | |
| M1 | 25 | 62.5 | |
| Tumor staging groups | |||
| I | Early stage | 6 | 15.0% |
| II | 2 | 5.0% | |
| III | Late stage | 7 | 17.5% |
| IV | 25 | 62.5% | |
| Liver infiltration | 32 | 80.0 | |
| Resectability | |||
| Yes | 8 | 20.0 | |
| No | 32 | 80.0 | |
Serum Levels of Cytokines in Gallbladder Cancer Patients and Healthy Controls
Serum IL-6 was significantly higher (49.13 [14.12 – 96.55] pg/mL) in GBC cases as compared to the control group (4.62 [3.44 – 5.77] pg/mL). However, TNF-α was found to be undetectable in the control group, so for statistical purposes, all the control group TNF-α values were taken as 0.09 pg/mL, which was the lower limit of detection of the kit we used. For the GBC group, only 42.5% of patients showed TNF-α in a detectable range, from 0.09 to 15.81 pg/mL. Outof the detectable patients, only five showed TNF-α at a higher level (Figure 1, Table 2). To determine the clinical utility of serum IL-6 and TNF-α, the ROC curve was plotted (Figure 2). ROC curve analysis revealed an area under curve (AUC) for serum IL-6 of 0.89 witha sensitivityof 82.5% andspecificityof 97.5% atanoptimal cutoff value of 13.49 pg/mL as determined by Youden’s index. AUC for TNF-α was found to be 0.71 with a sensitivity and specificity of 40.0 and 100%, respectively, and a cutoff value of 0.24 pg/mL, indicating the high discriminatory potential of these cytokines in identifying GBC patients (Table 3).
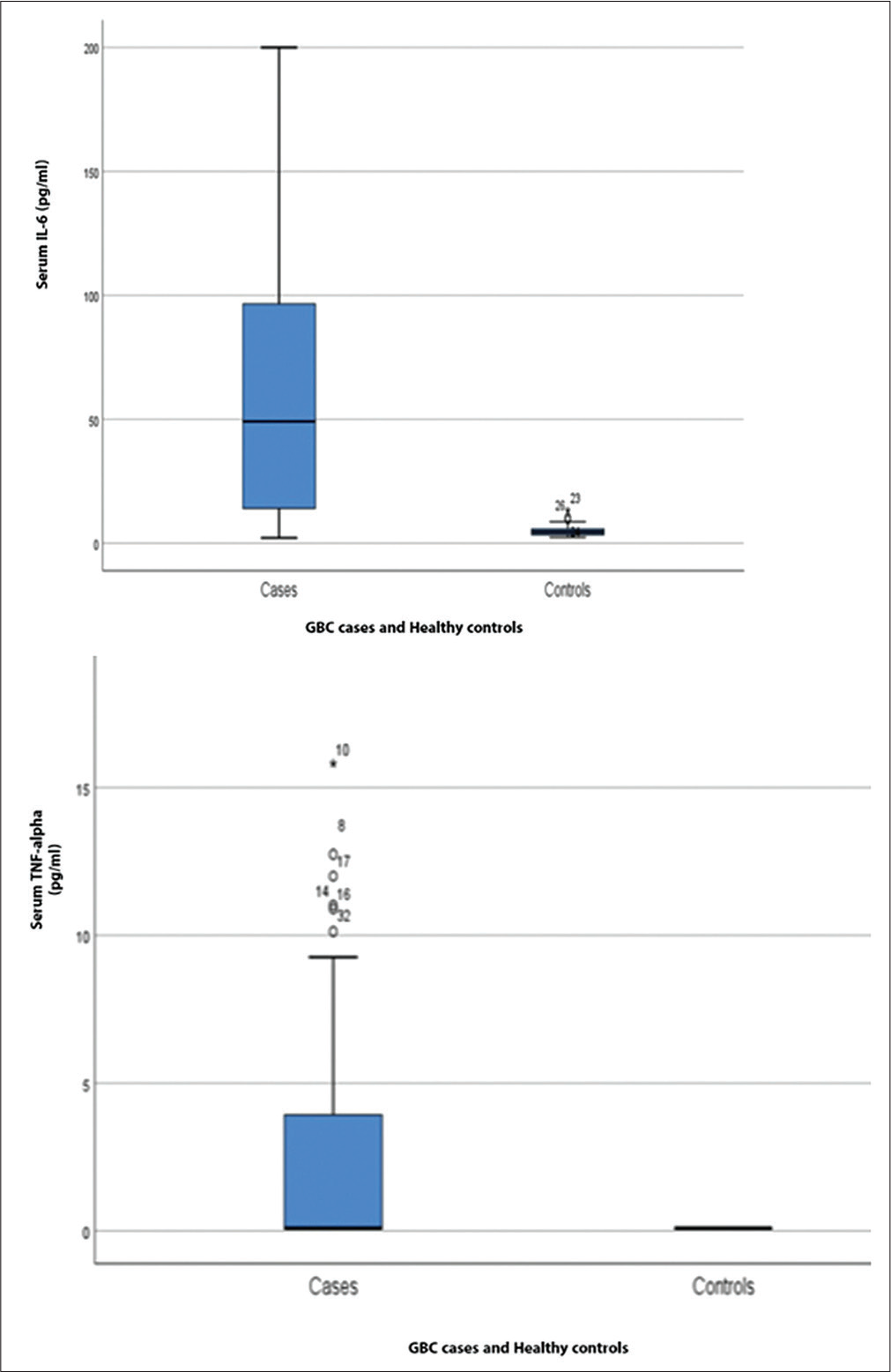
- Box plots demonstrating (a) serum IL-6 (values in pg/mL) and (b) TNF-α (values in pg/mL) in gallbladder carcinoma cases and healthy controls. IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
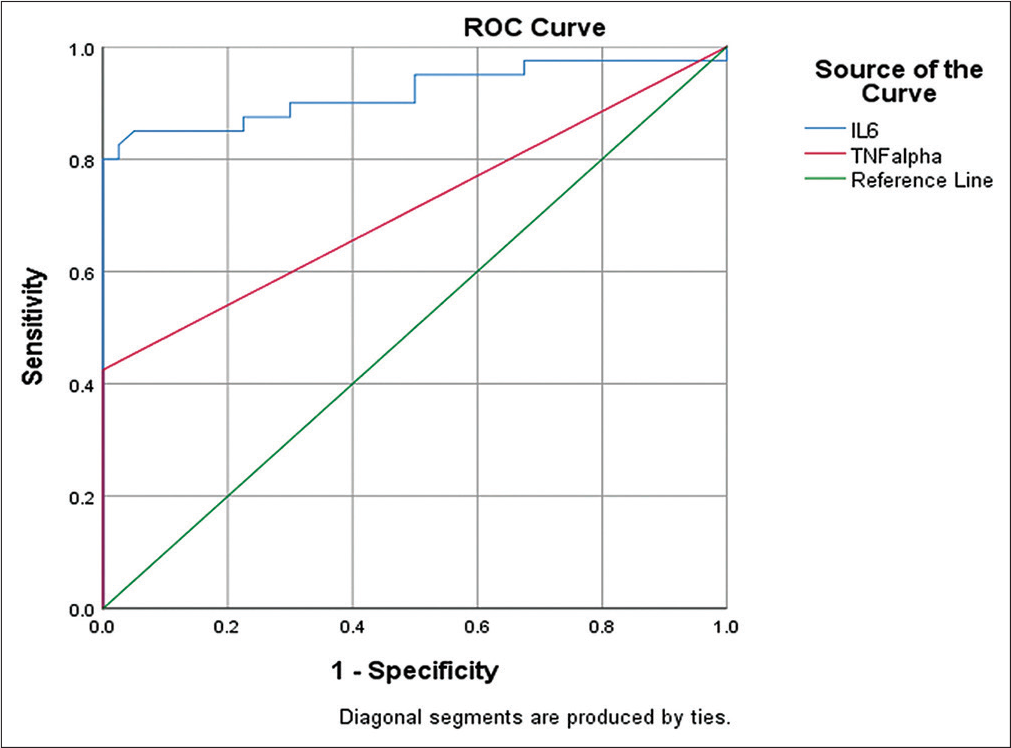
- ROC curves of serum IL-6 and TNF-α for the diagnosis of gallbladder cancer. IL-6, interleukin-6; receiver operating characteristics; TNF-α, tumor necrosis factor-alpha.
| Inflammatory marker | n | Z value | p-value |
|---|---|---|---|
| IL-6 (pg/mL) | |||
| GBC | 40 | –6.09 | 0.0001a |
| Controls | 40 | ||
| TNF-α (pg/mL) | |||
| GBC | 40 | –4.57 | 0.0001a |
| Controls | 40 |
Abbreviations: GBC, gallbladder cancer; IL-6, interleukin-6; TNF-κ, tumor necrosis factor-alpha.
aSignificant at the 0.05 level.
| AUC | Standard error | 95% confidence interval | Cutoff value (pg/mL) | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| IL-6 | 0.89 | 0.04 | 0.81 | 0.97 | 13.49 | 82.5 | 97.5 |
| TNF-α | 0.71 | 0.05 | 0.59 | 0.82 | 0.245 | 40.0 | 100 |
Abbreviations: AUC, area under the curve; IL-6, interleukin-6; ROC, receiver operating characteristics; TNF-α, tumor necrosis factor-alpha.
Comparison of Serum IL-6 and TNF-α with Clinicopathological Characteristics
The difference in serum IL-6 levels between the GBC resectable and nonresectable groups was statistically significant (p < 0.05). However, no statistically significant difference was observed for IL-6 levels with respect to metastasis, gallstones, lymph node involvement, or TNM (tumor size, node involvement, and metastasis status) stage in GBC patients. Serum TNF-α levels do not significantly vary among the different clinicopathological characteristics of GBC patients (Table 4). Both serum IL-6 and TNF-α were not found to be associated with age, gender, gallstones, total bilirubin, smoking, and alcoholism by univariate and multivariate logistic regression analyses.
| Clinicopathological characteristics | IL-6 | TNF-α | |||
|---|---|---|---|---|---|
| Median (IQR) | p-value | Median (IQR) | p-value | ||
| Metastasis | |||||
| Yes | 56.71 (17.80–96.54) | 0.31 | 0.24 (0.09–7.96) | 0.23 | |
| No | 47.55 (4.76–108.12) | 0.09 (0.09–1.50) | |||
| Gallstones | |||||
| Yes | 47.84 (14.27–88.98) | 0.50 | 0.09 (0.09–3.74) | 0.19 | |
| No | 65.66 (8.94–147.19) | 0.29 (0.09–11.36) | |||
| Resectability | |||||
| Yes | 5.12 (2.97–70.90) | 0.01a | 0.09 (0.09–9.02) | 0.59 | |
| No | 54.30 (20.81–117.19) | 0.09 (0.09–4.11) | |||
| Lymph node involvement | |||||
| Yes | 51.89 (17.80–114.91) | 0.06 | 0.09 (0.09–5.65) | 0.98 | |
| No | 5.49 (3.78–66.414) | 0.09 (0.09–3.56) | |||
| TNM stage | |||||
| Early | 20.85 (4.02–74.38) | 0.08 | 0.09 (0.09–9.89) | 0.88 | |
| Late | 54.30 (16.99–117.19) | 0.09 (0.09–3.59) | |||
| Liver infiltration | |||||
| Yes | 50.01 (14.86–117.19) | 0.27 | 0.09 (0.09–1.34) | 0.13 | |
| No | 44.31 (4.02–76.03) | 1.90 (0.09–11.53) | |||
Abbreviations: IL-6, interleukin-6; IQR, interquartile range; TNF-α, tumor necrosis factor-alpha; TNM, tumor size, node involvement, and metastasis status.
aSignificant at the 0.05 level.
Correlation of IL-6 and TNF-α with Serum Tumor Markers
A significant positive correlation was found between IL-6 and CA125 (p = 0.028, r = 0.348), while there was no significant correlation between TNF-α and tumor markers (Table 5).
| Tumor markers | Median (IQR) | (N = 40) | IL-6 | TNF-α |
|---|---|---|---|---|
| CEA (ng/mL) | 3.97 (2.42–26.86) | Correlation coefficient | 0.29 | 0.18 |
| p-value | 0.06 | 0.25 | ||
| CA125 (units/mL) | 67.05 (24.5–214.4) | Correlation coefficient | 0.34 | –0.06 |
| p-value | 0.02a | 0.70 | ||
| CA19-9 (IU/mL) | 84.21 (14.58–1,200) | Correlation coefficient | 0.24 | 0.05 |
| p-value | 0.12 | 0.73 |
aSignificant at the 0.05 level.
Evaluation of Risk Factors in GBC Patients
Univariate and multivariate logistic regression analyses revealed age, gender, gallstones, total bilirubin, smoking, and alcoholism were not risk factors for GBC (Table 6).
| Risk factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| Z score | p-value | Z score | p-value | |
| Age | –1.09 | 0.275 | –0.996 | 0.31 |
| Gender | 0.413 | 0.679 | 0.623 | 0.62 |
| Gallstones | –0.706 | 0.48 | 0.488 | 0.48 |
| Alkaline phosphatase | 0.974 | 0.33 | 0.270 | 0.27 |
| Total bilirubin | –0.045 | 0.964 | 0.075 | 0.94 |
| Smoking | 0.962 | 0.335 | 1.378 | 0.168 |
| Alcoholism | 0.006 | 0.995 | 0.006 | 0.995 |
DISCUSSION
GBC originates from the gallbladder epithelia, which are of highly malignant potential and the most lethal tumor among different biliary tract cancers worldwide.[1] GBC, owing to its high incidence, late-stage diagnosis, and aggressive tumor behavior with high morbidity and mortality, deserves extensive clinical research, which directs toward its early detection. The link between GBC and inflammation is well established, and the key endogenous promoters include inflammatory cytokines such as IL-1β, IL-6, and TNF-α, which are the major players in cancer-related inflammation.[20] The pathogenesis and advancement of GBC may be facilitated by regional inflammatory responses, which entail the discharge of IL-6 and TNF-α. These responses may cause genetic modifications that promote the survival and proliferation of mutated cells, impede apoptosis, and stimulate angiogenesis and metastasis.[20] Our study only focused on establishing the relationship between IL-6 and TNF-α with GBC.
In our investigation, the GBC patients’ serum IL-6 and TNF-α levels were found to be considerably higher than those of healthy controls. As IL-6 and TNF-α are known to be essential players in cancer-related inflammation, this is consistent with the findings of earlier studies and supports the link between inflammation and cancer. Similar research on colorectal cancer by Xu et al revealed a high serum IL-6 level linked to a poor prognosis for both colorectal cancer overall survival and disease-free survival, suggesting that serum IL-6 may be a viable biomarker for the detection of colorectal cancer.[21] In a different study on prostate cancer, Michalaki et al found significantly higher levels of IL-6 and TNF-α in metastatic diseases than in local illness.[7]
ROC curve was generated to determine the diagnostic efficacy or utility of IL-6 and TNF-α in GBC patients. At an ideal cutoff value of 13.49 pg/mL, the sensitivity and specificity of IL-6 were 82.5 and 97.5%, respectively, according to the ROC curve analysis. The cutoff value for TNF-α was 0.24 pg/mL, and the sensitivity and specificity were 40.0 and 100%, respectively. No control sample for TNF-α revealed concentrations above the detectable limit, demonstrating the great specificity of the test. IL-6 was found to be a more sensitive marker than TNF-α. However, it was uncertain if it had a diagnostic or predictive function.
There was also a significant difference in the levels of serum IL-6 between the groups of patients with resectable and nonresectable GBC. The median value of the nonresectable group was significantly higher than that of the resectable group, implying that as the disease progresses, the release of inflammatory markers also increases. According to Coussens and Werb’s work, inflammation plays a crucial role in the growth of malignancies, which helps explain our findings.[22] The study’s results indicate that the tumor microenvironment, which is primarily mediated and supported by inflammatory cells, is an essential component of the neoplastic process because it supports the growth, survival, and migration of cancer cells.[22] The lack of association with other factors such age, gender, gallstones, total bilirubin, smoking, and alcoholism might be the result of a small sample size or a skewed distribution of cases since more patients had cancer that was in an advanced stage.
On correlating serum IL-6 and TNF-α with serum tumor markers, we observed a significant positive correlation between IL-6 and CA125. This may be understood in light of a prior research by Rajput et al that showed a strong correlation between the increase of CA125 and IL-6 levels in individuals with benign and malignant ovarian tumors.[23]
In benign instances, levels of both biomarkers were almost normal, but the levels were elevated in malignant cases.[23]
However, IL-6 failed to show a correlation with CEA and CA19-9. In our study, TNF-α fails to show any association with tumor markers.
In our study, age, gender, gallstones, total bilirubin, smoking, and alcoholism were not found to be risk factors for GBC. This is contrary to earlier studies in which these were shown to be risk factors. This may be due to the low sample size and noninvolvement of all stages of GBC stages.
The current study’s limitation is the small sample size and restricted geographic dispersion. The serum levels of IL-6 and TNF-α as indicators of inflammation in other noncancerous gallbladder inflammatory disorders were not evaluated in this study. Additionally, fewer patients were in the early stages, so firm conclusions could not be drawn. Since the bulk of the case group is at an advanced stage, we need to conduct further research with larger sample sizes that include all GBC stages in order to comprehend the diagnostic and prognostic roles played by IL-6 and TNF-α.
CONCLUSION
Serum IL-6 may be utilized to diagnose GBC cases, predict whether a case would be amenable to resection, and identify instances of GBC in combination with other serum tumor markers. In contrast, serum TNF-α, despite its high area under the curve, showed low sensitivity for the detection of GBC. However, further studies encompassing noninflammatory conditions are required to make a conclusive remark on its utility as a specific biomarker.
Ethical Approval and Consent to Participate
Ethical approval was given by the All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India, under reference number 422/IEC/M. Sc/2020.
Conflict of Interest
None declared.
Funding
None.
References
- Geographic pathology revisited: development of an atlas of cancer in India. Int J Cancer. 2005;116:740-754.
- [CrossRef] [PubMed] [Google Scholar]
- Gallbladder cancer epidemiology, pathogenesis and molecular genetics: re-cent update. World J Gastroenterol. 2017;23:3978-3998.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gallbladder cancer: recog-nition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol. 2000;95:1402-1410.
- [CrossRef] [PubMed] [Google Scholar]
- Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation and withaferin A inhibited the signaling in colorectal cancer cells. Mediators Inflamm. 2017;2017:5958429.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312-2316.
- [CrossRef] [PubMed] [Google Scholar]
- Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer. 2013;132:1977-1985.
- [CrossRef] [PubMed] [Google Scholar]
- The significant role of interleukin-6 and its signaling pathway in the immunopatho-genesis and treatment of breast cancer. Biomed Pharmacother. 2018;108:1415-1424.
- [CrossRef] [PubMed] [Google Scholar]
- The role of interleukin-6 in the evolution of ovarian cancer: clinical and prognostic implications: a review. J Mol Med (Berl). 2013;91:1355-1368.
- [CrossRef] [PubMed] [Google Scholar]
- Author correction: circulating levels of inflammatory proteins and survival in patients with gallblad-der cancer. Sci Rep. 2020;10:2680.
- [CrossRef] [PubMed] [Google Scholar]
- Serum IL6 as a prognostic biomarker and IL6R as a therapeutic target in biliary tract cancers. Clin Cancer Res. 2020;26:5655-5667.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275-1288.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor necrosis factor-α promotes the lymphangiogenesis of gallbladder carcinoma through nuclear factor-κB-mediated upregulation of vas-cular endothelial growth factor-C. Cancer Sci. 2014;105:1261-1271.
- [CrossRef] [PubMed] [Google Scholar]
- TNF-alpha promotes lymphangio-genesis and lymphatic metastasis of gallbladder cancer through the ERK1/2/AP-1/VEGF-D pathway. BMC Cancer. 2016;16:240.
- [CrossRef] [PubMed] [Google Scholar]
- Do expression profiles of cytokines VEGF, TNF-α, IL-1ß, IL-6 and IL-8 correlate with gall-bladder cancer. Journal of Cancer Science and Clinical Oncology. 2015;2:2.
- [Google Scholar]
- Chronic inflammation and cytokines in the tumor microen-vironment. J Immunol Res. 2014;2014:149185.
- [CrossRef] [PubMed] [Google Scholar]
- The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751-762.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44:937-945.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic inflammation and gallbladder cancer. Cancer Lett. 2014;345:242-248.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine (Baltimore). 2016;95:e2502.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between serum levels of CA-125 and interleukin-6 with the degree of differentiation in ovarian neoplasms. Annals of Pathology and Laboratory Medicine. 2019;6:392-397.
- [CrossRef] [Google Scholar]





