Translate this page into:
Burkholderia vietnamiensis causing bacteremia in patients suffering from B-cell acute lymphocytic leukemia: A case series and review of literature
*Corresponding author: Sangram Singh Patel, MD, Department of Microbiology, C Block, 2nd Floor, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014, Uttar Pradesh, India sangramsgpgi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kar M, Dubey A, Sahu C, Patel SS. Burkholderia vietnamiensis causing bacteremia in patients suffering from B-cell acute lymphocytic leukemia: A case series and review of literature. J Lab Physicians. 2024;16:134-9. doi: 10.1055/s-0043-1774722
Abstract
Burkholderia cepacia complex consists of 24 species of microorganisms that include B. cepacia, B. multivorans, B. cenocepacia, B. vietnamiensis, B. ambifaria, and many more. It is a ubiquitous microorganism found in the soil and aquatic milieu. The probability of infections in immunocompetent individuals is rare, but an increase in the population of immunocompromised patients in the past decade has attributed to increasing incidence of infections caused by these microorganisms. The most common infections caused by these isolates include bacteremia, pneumonia, genital tract infections, and surgical wound infections. Their potential to cause nosocomial outbreaks in wards of immunocompromised patients is well known. In this case series, we describe three cases of community-acquired Burkholderia vietnamiensis bacteremia in patients suffering from B-cell acute lymphocytic leukemia and their antibiotic sensitivity pattern to guide the treatment of these individuals.
Keywords
Burkholderia cepacia complex (BCC)
hematological malignancies
immunocompromised individuals
bacteremia
Burkholderia vietnamiensis
INTRODUCTION
Burkholderia cepacia is a pathogenic gram-negative, aerobic bacillus usually isolated from the aquatic milieu. It is identified as B. cepacia complex (BCC), which consists of 24 species, of which the most commonly isolated are B. cepacia, B. multivorans, B. cenocepacia, B. vietnamiensis, B. ambifaria, B. anthina, B. pyrrocinia, B. ubonensis, B. dolosa, and B. stabiliz.[1,2] Bacteria belonging to the BCC group have recently emerged as the cause of opportunistic infections in immunocompromised individuals. The number of immunosuppressed individuals has increased in the wake of elderly debilitated patients, chronic kidney disease patients, cancer patients on chemotherapy, organ transplant patients, cystic fibrosis patients, and patients on immunosuppressant therapy, coronavirus disease 2019 infections, etc.[3–5]
Immunosuppression in the case of cancer patients undergoing chemotherapy may either be due to the nature of the disease or it may be caused by the treatment.[6] Several studies have reported the isolation of the opportunistic pathogen as an outbreak-causing agent while admitted to the wards due to contamination of drugs and intravenous fluids.[7] In this case series, we describe three cases of community-acquired Burkholderia vietnamiensis bacteremia in young patients of B-cell acute lymphocytic leukemia, who were admitted to a 1,600-bedded teaching hospital in Northern India.
Case 1
A 29-year-old female presented to the internal medicine outpatient department on March 6, 2022 with chief complaints of fever with chills and rigors, cough, and chest pain for the past 15 days. Her routine blood investigations showed a hemoglobin of 3 g/L, a total leukocyte count of 2,400 cells per cubic mm and a differential leukocyte count of neutrophil 68%, lymphocytes 29%, monocytes 2%, and eosinophils 1% suggestive of pancytopenia. Due to unusually low total leukocyte count, infective cause of fever was ruled out that was confirmed by a blood culture bottle sent to the department of microbiology on the day of presentation with fever that flagged negative after 5 days of incubation. She was referred to the hematology outpatient department where she was advised to undergo a bone marrow examination to know the cause of pancytopenia on March 22, 2022. Her bone marrow examination on hematoxylin and eosin staining was suggestive of almost complete replacement by small and mediumsized blast cells (Figure 1) without any family history of the same. The blast cells were myeloperoxidase negative. B-Cell lineage was identified by flow cytometry and she was diagnosed with B-cell type acute lymphocytic leukemia. The patient continued to have persistent fever and pleuritic chest pain, for which she was admitted to the inpatient department of hematology department. She underwent high-resolution chesttomography (HRCT) (Figure 2) onwhichnodular opacities were observed suggestive of fungal pneumonia for which liposomal amphotericin B was started on third day of admission, although no respiratory sample was sent to obtain any microbiological evidence of fungal pneumonia. She became afebrile for 2 days but started to develop fever again.
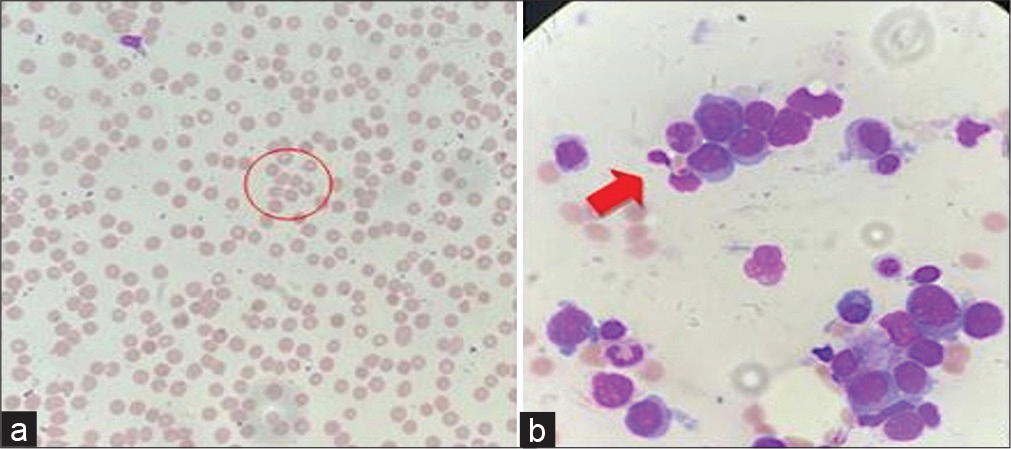
- Bone marrow examination of case 1 on hematoxylin and eosin staining was suggestive of almost complete replacement by smalland medium-sized blast cells (B-acute lymphoblastic leukemia). The circle and arrow represents the presence of immature red blood cells and white blood cells in the peripheral smear of the patients.
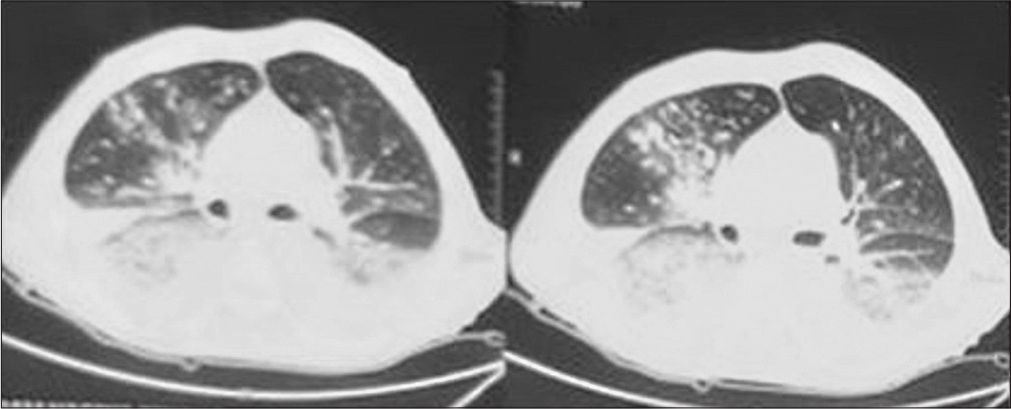
- High-resolution chest tomography of case 1 showing nodular opacities suspected of fungal pneumonia.
Blood sample inoculated in two pairs of aerobic and anaerobic BD BACTEC (automated blood culture system, Becton Dickinson and Company, Becton Drive, Franklin Lakes, NJ, United States) blood culture bottles and for procalcitonin assay were sent to the bacteriology section of the department of microbiology on the fifth day of admission. A pair of aerobic and anaerobic blood culture bottles flagged positive within 48 hours of incubation, followed by the second pair of blood culture bottles in the next 24 hours, thus confirming the pathogenic nature of the microorganism. A subculture from both pairs on MacConkey agar (Figure 3) and blood agar (Figure 4) showed nonfermenting colonies that were identified as Burkholderia vietnamiensis by matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS) assay (BioMérieux, France, Model no. VITEK MS, SN 51192) with a confidence value of 99.9% with the detection profile of MALDI-TOF-MS for B. cepacia complex that is more than 95.5% and Figure 5 represents the MALDI-TOF spectrum of B. vietnamiensis for this isolate. Her procalcitonin was 2.31 ng/mL, well above the cutoff value of 0.5 ng/mL performed by chemiluminescence method. Antibiotic sensitivity testing was performed for the same by the Kirby Bauer disc diffusion method in accordance with the Clinical and Laboratory Standards Institute (CLSI) guideline,[1] with sensitivity for levofloxacin interpreted using the zone-diameter break-points of Pseudomonas species and was found sensitive to imipenem and meropenem. She was started on intravenous meropenem-cilastatin combination 1 g every 8 hours for 7 days following the antibiotic sensitivity testing and her fever started responding after 7 days of starting the antibiotics along with amphotericin B. A repeat blood culture was sent on the 7th day of antibiotic therapy and was reported sterile and repeated procalcitonin levels were reduced to 0.15 ng/mL. She was started on dasatinib and dexamethasone as per hospital protocol and tolerated this therapy well with no fever spikes or severe cytopenia. As her total leukocyte count was below 10,000/µL, she was started on dasatinib 50 mg once daily (OD) and was not increased further due to known interaction with antifungal voriconazole that was stepped down after the course of liposomal amphotericin B for radiologically confirmed fungal pneumonia. Her family was counseled for her need for a bone marrow transplant. She was lost to follow-up in the hematology outpatient department after 15 days of discharge.
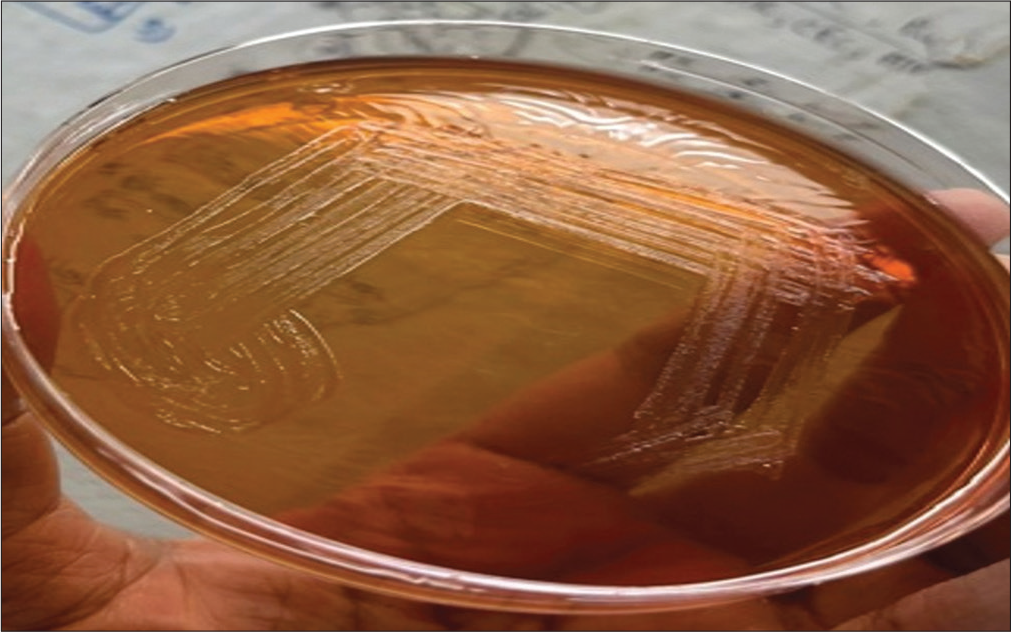
- A subculture from a blood culture bottle on MacConkey agar showing non-lactose fermenting colonies.
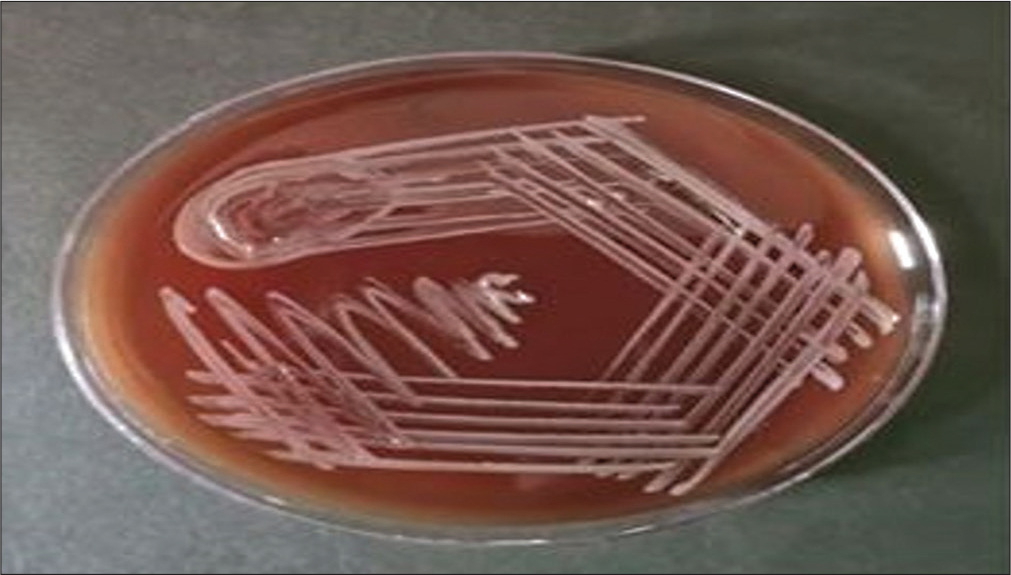
- A subculture from a blood culture bottle on blood agar showing hemolytic colonies on blood agar.
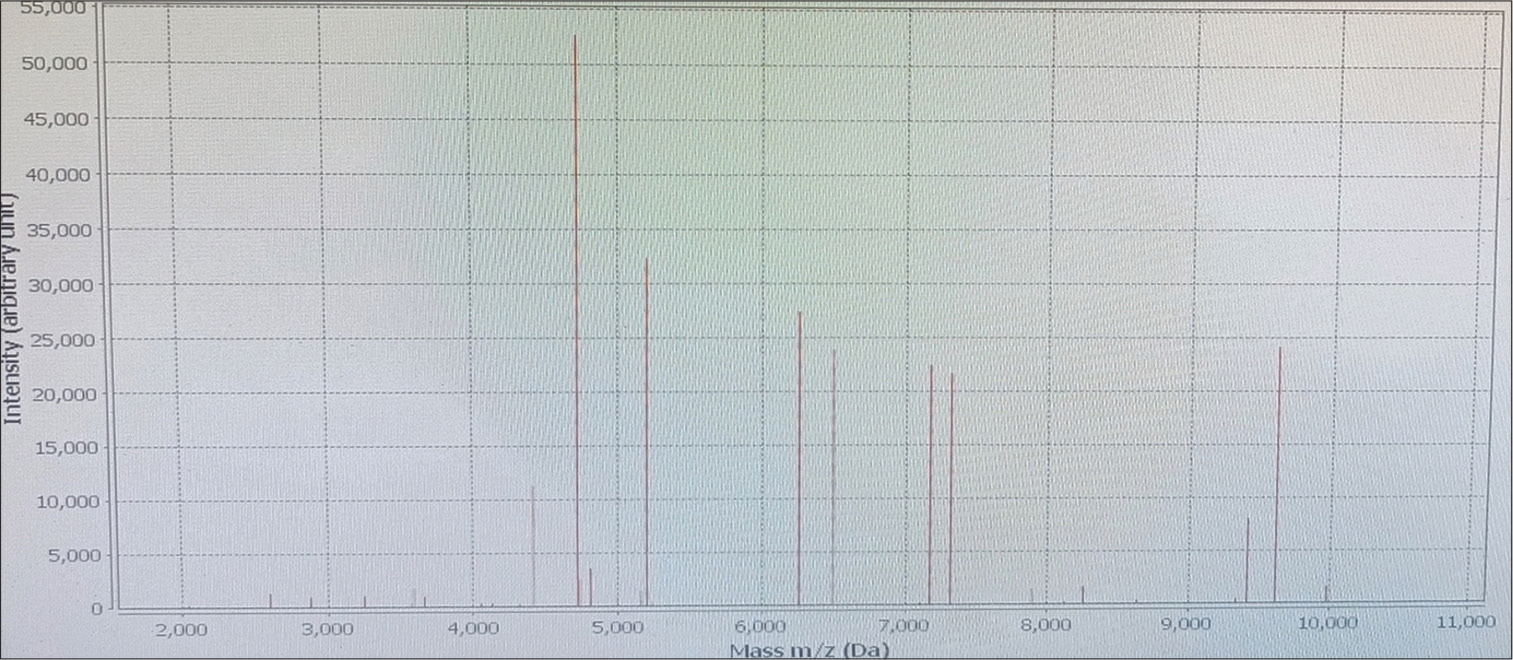
- Matrix-assisted laser desorption/ionization-time of flight spectrum of Burkholderia vietnamiensis isolate identified from case 1.
Case 2
A 22-year-old man presented to the internal medicine outpatient department on January 8, 2022 with chief complaints of generalized weakness and pain in the upper limb for the past 15 days. He was advised for routine blood investigation that revealed cytopenia with hemoglobin of 4 g/L, a total leukocyte count of 4,800 cells per cubic mm, and a differential leukocyte count of neutrophil 70%, lymphocytes 27%, monocytes 2%, and eosinophils 1% and was referred to the hematology outpatient department where his bone marrow examination performed on January 24, 2022 revealed B-cell acute lymphoblastic leukemia on hematoxylin and eosin staining. No family history of any hematological malignancies was reported. The patient came for a follow-up to the outpatient department within 15 days with high-grade fever and vomiting. On evaluation, he was found to have a non-neutropenic fever. His chest X-ray was normal. According to standard protocol for diagnosis of sepsis, blood samples were sent for procalcitonin assay performed by chemiluminescence method and a pair of BACTEC blood cultures was raised and sent to the bacteriology section of the department of microbiology. His procalcitonin was 36 ng/mL and his BACTEC blood culture flagged positive after 48 hours of incubation. On subculture, MacConkey agar revealed growth of nonlactose fermenting, gram-negative bacilli blood agar showed hemolytic colonies that was identified as B. vietnamiensis by MALDI-TOF-MS assay (BioMérieux, France) with a confidence value of 98.9% with the detection profile of MALDI-TOF-MS for B. cepacia complex that is more than 95.5%. On antibiotic sensitivity testing by Kirby–Bauer disc diffusion method in accordance with the CLSI guidelines,[1] with sensitivity for levofloxacin interpreted using the zone-diameter breakpoints of Pseudomonas species and the isolate was found sensitive to meropenem and levofloxacin. The patient was started on intravenous meropenemcilastatin combination 600 mg every 6 hours for 10 days according to the antibiotic susceptibility and the patient started torespond totreatment. His feverand vomiting subsided after 48 hours of antibiotic administration. His procalcitonin dropped to 2 ng/mL after the progress of treatment, while a pair of repeat BD BACTEC blood cultures sent to the microbiology department after 7 days of antibiotic treatment was reported sterile after 5 days of incubation. The intravenous antibiotics were continued for 10 days and the patient was further started on tab. prednisolone 100 mg OD and folic acid 5 mg OD as per protocol of the hospital. He was discharged in stable condition and was advised to continue chemotherapy as per schedule. He presented to the outpatient department after 15 days of discharge, devoid of any symptoms of infection, and was allocated his schedule for next chemotherapy session.
Case 3
A 5-year-old boy presented to the pediatric outpatient department on May 15, 2022 with chief complaints of fever and generalized weakness for the past 10 days. His hemoglobin was 3.6 g/L, a total leukocyte count of 1,800 cells per cubic mm, and a differential leukocyte count of neutrophil 62%, lymphocytes 36%, monocytes 1%, and eosinophils 1% suggestive of pancytopenia. His fever subsided on administration of oral antipyretics and a baseline blood culture sample in BACTEC blood culture bottle sent to the department flagged negative after 5 days of incubation and he was referred to the hematology outpatient department where hematoxylin and eosin staining of his bone marrow examination performed on May 29, 2022 revealed B-cell acute lymphoblastic leukemia without any family history of the same. On further evaluation, his HRCT chest was within normal limits. According to standard protocol for diagnosing sepsis, blood sample was drawn for procalcitonin assay and a pair of BACTEC blood cultures was sent to the bacteriology section of the department of microbiology. His procalcitonin performed by chemiluminescence method was 6.50 ng/mL and BACTEC blood culture flagged positive after 72 hours of incubation. On subculture, MacConkey agar revealed growth of nonlactose fermenting; gram-negative bacilli blood agar showed hemolytic colonies that was identified as B. vietnamiensis by MALDI-TOFMS assay (BioMérieux, France) with a confidence value of 95.84% with the detection profile of MALDI-TOF-MS for B. cepacia complex that is more than 95.5%. Onantibiotic sensitivity testing by Kirby–Bauer disc diffusion method in accordance with the CLSI guidelines,[1] with sensitivity for levofloxacin interpreted using the zone-diameter breakpoints of Pseudomonas species and theisolate wastestedfor andwasfound sensitive tociprofloxacin, meropenem, and minocycline. The patient was started on intravenous meropenem-cilastatin combination 600 mg every 6 hour for 10 days and after 3 days of treatment the patient was afebrile, although he still complained of generalized weakness. Patient wascontinuedonintravenousantibioticsandwasfurtherstarted on folic acid syrup OD. He was discharged in stable condition and came for follow-up after 21 days and was advised to continue chemotherapy as per schedule.
DISCUSSION
BCC consists of a species named B. vietnamiensis that was the causative microorganism, identified from the blood culture of two patients suffering from bacteremia and impending sepsis. Burkholderia species naturally occur in the environment, especially in the aquatic milieu.[2] It is an aerobic, gram-negative bacillus that was found responsible for causing an array of infections in immunocompromised patients.[3]
The number of immunosuppressed individuals has increased in the wake of increasing human immunodeficiency virus-positive cases, elderly debilitated patients, chronic kidney disease patients, cancer patients on chemotherapy, organ transplant patients, cystic fibrosis patients, and patients on immunosuppressant therapy, etc.[4–6] Bacteria belonging to the BCC grouphave recently emerged as thecause of opportunistic infections in immunocompromised individuals. Immunosuppression in the case of cancer patients undergoing chemotherapy may either be due to the nature of the disease or it may be caused by the treatment.[7] Several studies have reported the isolation of theopportunisticpathogenas anoutbreak-causing agent while admitted to the wards due to contamination of drugs and intravenous fluids.[8] It was predominantly reported as a respiratory pathogen in cystic fibrosis patients by Ashour and El-Sharif but can also cause infections in immunosup-pressed patients like those suffering from chronic granulomatous disease, hematological malignancies, and solid tumors.[9]
BCC is the probable causative agent causing bacteremia, pneumonia, genital tract infections, and surgical wound infections.[10] A review of recently reported cases of B. vietnamiensis infections in literature is mentioned in Table 1. Cases of BCC bacteremia were reported in immunocompromised patients in several cases but most were identified as an outbreak of nosocomial infections among patients admitted to a particular hospital. A study conducted by Singhal et al reported an outbreak of bacteremia among cancer patients admitted to a tertiary care center due to an infected antiemetic solution.[8]
| Sl.no. | Author name | Age and gender of patients | Year of publication | Place | Symptoms | Radiological investigations | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Magalhães et al. [11] |
23 months/ male |
2002 | Brazil, South America | Know case of cystic fibrosis with acute bronchitis | NA | Acute bronchitis caused by Burkhol-deria vietnamiesis | Ciprofloxacin, cotrimoxazole, and amikacin | Alive |
| 2. | Carvalho et al.[12] |
13 years/male | 2005 | Brazil, South America | Know case of cystic fibrosis for evalua-tion of his sputum sample for 9 years | NA | Chronic respiratory infection of a cystic fibrosis patient with B. vietnamiensis | NA | Alive |
| 3. | Marquette et al.[13] |
29 years/male | 2015 | Cambridge, United Kingdom | Know case of cystic fibrosis on a prophylactic treat-ment using temocillin | NA | Prophylactic treat-ment regimen against B.vietnamiensis in Cystic fibrosis patients | Nebulized temocillin | Alive |
| 4. | Zhang et al.[14] |
71 years/male | 2018 | Hangzhou, Zhejiang, P. R. China | Complaint of episodic fever for 1 week, accompa-nied by general malaise, nausea, and vomiting | Abdominal CT scans revealed a 5 cm abscess located in liver segment IV | Hepatic rupture due to purulent infection caused by B. vietnamiensis in a diabetic patient |
Cefoperazone and ornidazole | Alive |
| 5. | Spoletini et al.[15] |
22 years/female | 2019 | Leeds, United Kingdom | Lung transplant prospect with bacterial coloniza-tion and pulmonary exacerbation | NA | Pulmonary exacer-bation with B. viet-namiensis in organ transplant recipient | Minocyline and ceftazidime-avibactam | Alive |
| 6. | Rohit et al.[16] |
33 years /female |
2020 | Tamil Nadu, India | Large breast abscess | pus was collected by ultrasound guided aspiration | Purulent skin infec-tion caused by B. vietnamiensis | Levofloxacin | Alive |
| 7. | This case | Age of the patients for |
2022 | Lucknow, Uttar Pradesh, India | Fever, malaise, cough with expec-toration in B-cell acute lymphocytic leukemia patients | Bilateral patchy consolidation in the first case of the series |
B. cepacia complex bacteremia. We found 3 cases of B. vietnamiensis in patients suffering from B-ALL patients |
Levofloxacin ceftazidime, meropenem, cotrimoxazole, and minocycline | First patient was lost to follow-up and the other two were alive |
Abbreviations: B-ALL, B-acute lymphoblastic leukemia; CT, computed tomography; NA, not available.
The identification of community-acquired BCC has rarely been reported, but their existence in the soil and water where the microorganism is acted upon by a plethora of plant metabolites renders it intrinsically resistant to anti-pseudomonal penicillins, first and second-generation cephalosporins, and aminoglycosides.[2] The isolates identified by us in our cases were susceptible to carbapenem and fluoroquinolones, but in all three cases the patients were symptomatic before admission and the BACTEC blood culture from all three patients readily recovered the isolate on subculture on blood and MacConkey agar.
Environmental, drug, and medicated fluid surveillance can yield the source of an outbreak for these rare opportunistic isolates in the hospital setting, as has been performed by Mihaila and Blaga,[7] Singhal et al,[8] and Tavares et al.[17] Due to the lack of community environmental surveillance measures, we could not identify the source of infection and the mechanism of acquiring the infection, but the study makes it clear that immunosuppressed individuals are susceptible to acquiring such rare infections from the community. We endorse the use of MALDI-TOF assay for its ability to differentiate among all 24 species of BCC up to 100% for its identification. The isolation of rare microorganisms like B. vietnamiensis needs to be discussed more frequently to know and frame the appropriate treatment for them.
CONCLUSIONS
The identification of rare opportunistic pathogens like B. vietnamiensis and adequate treatment in the pandemic-ridden era is the need of the hour due to a rise in immunocompromised conditions owing to the rigorous use of antimicrobials and steroids. Its intrinsic resistance to first and second-generation cephalosporins and polymyxin can cause a state of alarm during treatment via empirical antibiotics. Thus, the MALDI-TOF-MS assay helps direct the appropriate therapy to the patients.
Acknowledgment
Bone marrow smears provided by courtesy of the Department of Hematology at our institute.
Authors’ Contributions
Mitra Kar, Akanksha Dubey, and Sangram Singh Patel helped in protocol development. Akanksha Dubey and Mitra Kar helped in data collection. Chinmoy Sahu and Sangram Singh Patel helped in data analysis and supervision. Mitra Kar and Akanksha Dubey contributed towriting of the manuscript. Mitra Kar, Akanksha Dubey, Chinmoy Sahu, and Sangram Singh Patel reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest
None declared.
Funding
None.
References
- Clinical and Laboratory Standards Institute: Performance standards for antimicrobial susceptibility testing: 32nd Edition CLSI document M100 2022.
- [Google Scholar]
- Burkholderia cepacia complex: beyond pseudomonas and acinetobacter. Indian J Med Microbiol. 2011;29:4-12.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of misidentifications of species from the Burkholderia cepacia com-plex and description of a new member, the soil bacterium Burkholderia catarinensis sp. nov. Pathog Dis. 2017;75
- [CrossRef] [PubMed] [Google Scholar]
- Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: epidemiology, clonal variation, and genome-wide expression alterations. Front Cell Infect Microbiol. 2011;1:12.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of Burkholderia cepacia complex species among isolates recovered from per-sons with or without cystic fibrosis. J Clin Microbiol. 2005;43:2926-2928.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of Burkholderia dolosa on lung function and survival in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:421-425.
- [CrossRef] [PubMed] [Google Scholar]
- Burkholderia cepacia septicemia in a patient with acute myeloid leukemia in postchemotherapy bone marrow aplasia. Pak J Med Sci. 2013;29:1275-1277.
- [CrossRef] [PubMed] [Google Scholar]
- Outbreak of Burkholderia cepacia complex bacteremia in a chemotherapy day care unit due to intrinsic contamination of an antiemetic drug. Indian J Med Microbiol. 2015;33:117-119.
- [CrossRef] [PubMed] [Google Scholar]
- Species distribution and antimicrobial susceptibility of gram-negative aerobic bacteria in hospitalized cancer patients. J Transl Med. 2009;7:14.
- [CrossRef] [PubMed] [Google Scholar]
- Hospital-acquired infections related to contaminated substances. J Hosp Infect. 2007;65:15-23.
- [CrossRef] [PubMed] [Google Scholar]
- Burkholderia cepacia genomovar III and Burkholderia vietnamiensis double infection in a cystic fibrosis child. J Cyst Fibros. 2002;1:292-294.
- [CrossRef] [PubMed] [Google Scholar]
- Transient isolation of Burkholderia multivorans and Burkholderia cenocepacia from a Brazilian cystic fibrosis patient chronically colonized with Burkholderia vietnamiensis. J Cyst Fibros. 2005;4:267-270.
- [CrossRef] [PubMed] [Google Scholar]
- 319 Nebulised temocillin for prophylaxis against Burkholderia vietnamiensis in an adult with cystic fibrosis. J Cyst Fibros. 2015;14:S140.
- [CrossRef] [Google Scholar]
- Hepatic rupture: a case report of a severe complication of percutaneous catheter drain-age. Medicine (Baltimore). 2018;97:e9499.
- [CrossRef] [PubMed] [Google Scholar]
- Use of ceftazidime/avibactam for the treatment of MDR Pseudomonas aerugi-nosa and Burkholderia cepacia complex infections in cystic fibrosis: a case series. J Antimicrob Chemother. 2019;74:1425-1429.
- [CrossRef] [PubMed] [Google Scholar]
- Burkholderia vietnamiensis causing a non-lactational breast abscess in a non-cystic fibrosis patient in Tamil Nadu, India. Indian J Med Microbiol. 2020;38:496-499.
- [CrossRef] [PubMed] [Google Scholar]
- Burkholderia cepacia complex bacteria: a feared contamination risk in water-based pharmaceutical products. Clin Microbiol Rev. 2020;33:e00139-e19.
- [CrossRef] [PubMed] [Google Scholar]






