Translate this page into:
Coexpression of MYC and BCL2 oncoproteins in primary nodal versus primary extranodal diffuse large B-cell lymphoma
*Corresponding author: Nisha Modi, Department of Pathology, Sri Aurobindo Medical College and PGI, Indore, Madhya Pradesh, India. nishamodib@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maru S, Modi N, Varma A, Goel S, Karmarkar S, Ahuja S. Coexpression of MYC and BCL2 oncoproteins in primary nodal versus primary extranodal diffuse large B-cell lymphoma. J Lab Physicians. 2024;16:372-78. doi: 10.25259/JLP_26_2024
Abstract
Objectives:
Diffuse large B-cell lymphoma (DLBCL) is a morphologically and molecularly diversified disease with aggressive biological behavior. The double expression of MYC/BCL2 proteins portends a poorer prognosis. This study aims to evaluate the frequency, describe the clinicopathological features of the double-expressor phenotype of DLBCL in primary nodal (PN) versus primary extranodal (PEN) sites, and investigate their associations.
Materials and Methods:
A total of 48 patients with the double-expressor phenotype of lymphoma (DEPL) in a tertiary care hospital were included over three years. Clinicopathological parameters and associations were investigated based on the primary site.
Statistical Analysis:
Data were documented and analyzed using appropriate statistical tests.
Results:
The incidence of DEPL in our study was 28.7%. The median age of all DEPL patients was 56 years, with a predominance of men (69%). DEPL cases were further subcategorized as PN-DEPL (n = 33) and PEN-DEPL (n=15). Males were affected almost equally in both groups. More PN-DEPL patients exhibited B symptoms (82%), elevated lactate dehydrogenase (LDH) levels (73%), III/IV stage disease (71%), and maximum revised international prognostic index (R-IPI) score (64%) compared to PEN-DEPL patients. On the other hand, bone marrow (BM) involvement (87%), activated B-cell-type phenotype (80%), pathologic stage I/II (67%), and Ki67 index >90% (93%) were more common in PEN-DEPL patients.
Conclusions:
Significant differences were observed between PN-DEPL and PEN-DEPL in terms of B symptoms, LDH levels, stage at presentation, BM involvement, pathological subtype, Ki67 index, and R-IPI score. This study provides an estimate of the burden of this aggressive entity and encourages further prognostic studies and therapeutic trials.
Keywords
Double expressor
BCL2
MYC
Diffuse large B-cell lymphoma
World Health Organization classification
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is a clonal neoplasm distinguished by the heterogeneity of large B lymphoid cells, presenting distinct morphological subgroups and molecular variants based on gene expression profiling.[1] Cytogenetic studies have classified DLBCL based on the presence of MYC (8q24), BCL2 (18q21), and BCL6 (3q27) rearrangements, indicating a more aggressive clinical course.[1] The 5th edition of the World Health Organization (WHO) classification of hematolymphoid tumors recognizes a prognostic subset, high-grade B-cell lymphoma with MYC and BCL2 and BCL6 rearrangements, also known as double-hit/triple-hit lymphomas (DHL/THL).[2] While not a separate diagnostic entity in the WHO Blue Book, lymphomas coexpressing MYC and BCL2 oncoproteins by immunohistochemistry (IHC) are designated as double-expressor phenotype lymphomas (DEPL), which have consistently portrayed poorer outcomes than non-double-expressor phenotype DLBCL in various studies.[3,4] Notably, the clinical behavior of DEPL is relatively better than that of DHL/THL.[5,6]
To date, the frequency and clinicopathological parameters of DEPL patients remain poorly characterized. [7,8] This study aims to elucidate the distribution and clinicopathological associations of DEPL in primary nodal (PN) versus primary extranodal (PEN) sites, contributing to a deeper understanding of disease trajectory in DEPL patients depending on the primary sites.
MATERIALS AND METHODS
Study methodology
Ethical approval was obtained from the Institutional Review Board and Ethics Committee for this observational, retrospective, and single-center study conducted under resource-limited settings. The study period spanned three years, from October 2021 to September 2023, at the Department of Pathology. Due to the relative rarity of DEPL cases, a convenient sampling method was used.
Pathology records of all newly diagnosed DLBCL patients confirmed by IHC were retrieved. Hematoxylin-eosin-stained slides and IHC slides were thoroughly reviewed. Molecular studies were not performed due to a lack of facilities at our institute and resource constraints.
Definition of DEPL
Lymphomas coexpressing MYC and BCL2 proteins by IHC were defined as DEPL. IHC utilized the Ventana immunostaining system (Benchmark GX) and BioGenex ready-to-use antibodies (Anti-c-MYC clone, EP121, and Anti-Human BCL-2 Alpha clone, SP66) employing heat-induced epitope retrieval. Cutoff values for MYC and BCL2 protein expression were decided at 40% and 50%, respectively, in accordance with most published studies. [1,8] Positive staining included moderate to strong nuclear staining for both proteins. DEPL cases were re-classified based on their primary sites as PN-DEPL and PEN-DEPL.
Inclusion criteria
Patients eligible for inclusion in this study were those diagnosed with DLBCL who meet the criteria for the DEPL, as defined previously. Included cases were newly diagnosed or de novo DLBCL instances, with tumors providing sufficient histological material for detailed analysis. In addition, the inclusion criteria required cases to have comprehensive and adequate clinical details available for a thorough examination.
Exclusion criteria
On the other hand, cases were excluded from the study if they involved DLBCL associated with distinct disease entities as per the WHO classification.[1,2] Furthermore, cases lacking sufficient histological material for proper analysis, those without essential clinical details, and instances involving the pediatric population (individuals under the age of 18) were excluded from the study. In addition, cases of relapsed DLBCL were not considered for inclusion in this study.
Parameters analyzed in DEPL, PN-DEPL, and PEN-DEPL are presented in Table 1.
| S. No | Parameters | Subcategories |
|---|---|---|
| I | Age | <60 or >60 years |
| II | Sex | male or female |
| III | B symptoms[1] | absent or present |
| IV | ECOG-PS[16] | “0 - Patient with no symptoms 1 - Patient with symptoms but is ambulatory 2 - Patient is bedridden for less than half the day 3 - Patient is bedridden half the day or longer 4 - Patient is chronically bedridden and requires assistance with the activities of daily living” |
| V | LDH level | Normal (140–280 U/L) or elevated |
| VI | Ann Arbor stage[1] | “Stage I - disease involving a single node or group of nodes Stage II - disease in more than one site – all lesions either below or above the diaphragm Stage III - disease on both sides of the diaphragm Stage IV - widespread involvement of extra lymphoid sites±lymph node involvement” |
| VII | BM involvement | Absent or present |
| VIII | Pathological subtype[1] | GCB type or ABC-type or unclassifiable “All the cases were subcategorized using the Hans algorithm which utilized 3 IHC markers; CD10, BCL6, and IRF4/MUM1, each considered positive if ≥30 percent of tumor cells stained positive.[1]Strong membranous staining for CD10 and strong nuclear expression was considered positive for BCL6 and MUM1.” |
| IX | Ki67 labeling index | ≤90 or >90 percentage; Ki-67 is a surrogate marker of proliferation. Any intensity of nuclear staining was considered positive. |
| X | Revised International Prognostic Score (R-IPI) score[16] | “≤3 or 4 or 5; R-IPI incorporates 5 clinical parameters: age, number of extranodal sites, LDH level, ECOG-PS, and pathologic stage.” Required data were recorded and an online calculator was used for score calculation.[16]It is an easy and valid tool for prognostic stratification. |
ECOG-PS: Eastern cooperative oncology group performance status, LDH: Lactate dehydrogenase, GCB: Germinal center B-cell, ABC: Activated B-cell, IHC: Immunohistochemistry, R-IPI: Revised international prognostic index
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences. Categorical variables were depicted as frequency and percentages. Intergroup comparison was done using the Chi-square test and Fisher’s exact test. P < 0.05 was considered significant. Graphical representations were done using bar charts, wherever appropriate.
RESULTS
Primary site distribution of DEPL
Out of 223 IHC-confirmed DLBCL cases, 56 were excluded due to a lack of adequate clinical information or histologic material, and 119 cases failed to co-express MYC/BCL2 proteins. Thus, 48 out of 167 evaluable cases (28.7%) of DEPL, as defined previously, were included in the study. DLBCL arising from sites other than the lymph nodes was classified under PEN-DEPL.
Among these DEPLs, 69% were detected in nodal sites, while 31% were categorized as PEN-DEPL. Extranodal sites involved included the large intestine (n = 4, 2.39%), stomach (n = 2, 1.19%), liver (n = 1, 0.6%), ovary (n = 1, 0.6%), tonsil (n = 1, 0.6%), salivary gland (n = 1, 0.6%), skin (n = 1, 0.6%), testis (n = 1, 0.6%), soft palate (n = 1, 0.6%), and retroperitoneum (n = 2, 1.19%)
Clinicopathologic features
The clinicopathologic profiles of DEPL, PN-DEPL, and PEN-DEPL patients are summarized in Table 2. The median age of DEPL patients was 56 (range, 41−86) years, with a male predominance (69%). The majority presented with B symptoms (65%), higher eastern cooperative oncology group performance status (ECOG-PS) (67%), elevated lactate dehydrogenase (LDH) levels (56%), higher-stage disease (71%), bone marrow (BM) involvement (56%), and maximum revised international prognostic index (R-IPI) score (50%). Of the 48 cases, the majority were of the pathological activated B-cell (ABC) type (46%) with a Ki67 index >90% (60%).
| Parameters | Subcategories | DEPL n(%) | PN-DEPL n(%) | PEN-DEPL n(%) | P-value |
|---|---|---|---|---|---|
| Total cases | 48 | 33 (69) | 15 (31) | ||
| Age | ≤60 | 20 (42) | 17 (51) | 3 (20) | 0.0592 |
| >60 | 28 (58) | 16 (49) | 12 (80) | ||
| Sex | Male | 33 (69) | 25 (76) | 8 (53) | 0.1799 |
| Female | 15 (31) | 8 (24) | 7 (47) | ||
| B symptoms | absent | 17 (35) | 6 (18) | 11 (73) | 0.0007 |
| present | 31 (65) | 27 (82) | 4 (27) | ||
| ECOG | 0-1 | 16 (33) | 8 (24) | 8 (53) | 0.0963 |
| ≥2 | 32 (67) | 25 (76) | 7 (47) | ||
| LDH | normal | 21 (44) | 9 (27) | 12 (80) | 0.0013 |
| elevated | 27 (56) | 24 (73) | 3 (20) | ||
| Stage | I /II | 14 (29) | 4 (12) | 10 (67) | 0.0003 |
| III/IV | 34 (71) | 29 (88) | 5 (33) | ||
| BM involvement | No | 21 (44) | 19 (58) | 2 (13) | 0.0051 |
| Yes | 27 (56) | 14 (42) | 13 (87) | ||
| Subtype | GCB | 17 (35) | 15 (45) | 2 (13) | 0.005916 |
| ABC | 22 (46) | 10 (30) | 12 (80) | ||
| unclassifiable | 9 (19) | 8 (25) | 1 (7) | ||
| Ki67 labeling index | ≤90 | 19 (40) | 18 (55) | 1 (7) | 0.0016 |
| >90 | 29 (60) | 15 (45) | 14 (93) | ||
| R-IPI score | ≤3 | 5 (10) | 3 (9) | 2 (13) | 0.017005 |
| 4 | 19 (40) | 9 (27) | 10 (67) | ||
| 5 | 24 (50) | 21 (64) | 3 (20) |
The figures in bold indicate significant associations. DEPL: Double-expressor phenotype lymphoma, PN-DEPL: Primary nodal double-expressor phenotype lymphoma, PEN-DEPL: Primary extranodal double-expressor phenotype lymphoma, N: Number, ECOG: Eastern cooperative oncology group, LDH: Lactate dehydrogenase, GCB: Germinal center B-cell, ABC: Activated B-cell, BM: Bone marrow, R-IPI: Revised international prognostic index
The incidence of PN-DEPL was higher compared to PENDEPL. The median ages of patients with PN-DEPL and PEN-DEPL were 56 and 61 years, respectively. Males were affected almost equally in both groups. B symptoms, LDH level, disease stage, BM involvement, pathological subtype, Ki67 index, and R-IPI score were compared between PNDEPL and PEN-DEPL patients. PN-DEPL patients had a higher incidence of B symptoms (82%), elevated LDH levels (73%), III/IV stage disease (71%), and maximum R-IPI score (64%). In contrast, BM involvement (87%), ABC-type phenotype (80%), disease stage I/II (67%), and Ki67 index >90% (93%) were more common in PEN-DEPL patients. The immunohistochemical features of PN-DEPL and Germinal center B-cell (GCB) type are illustrated in Figure 1, where large monomorphic atypical cells [Figure 1a] are showing positive expression for CD20 [Figure 1b], CD10 [Figure 1c], and BCL2 [Figure 1d]. Figure 2 displays the morphological and IHC findings of PEN-DEPL (skin), ABC type, where sheets of atypical lymphoid infiltrates are seen in the dermis [Figure 2a], showing immunonegativity for CD3 [Figure 2b], strong immunoexpression for CD20 [Figure 2c], and BCL2 [Figure 2d]. A higher power view of the same is depicted in Figure 3, where large tumor cells with non-cleaved nuclei are seen [Figure 3a] with immunopositivity for CD20 [Figure 3b], MYC [Figure 3c], and BCL2 [Figure 3d].
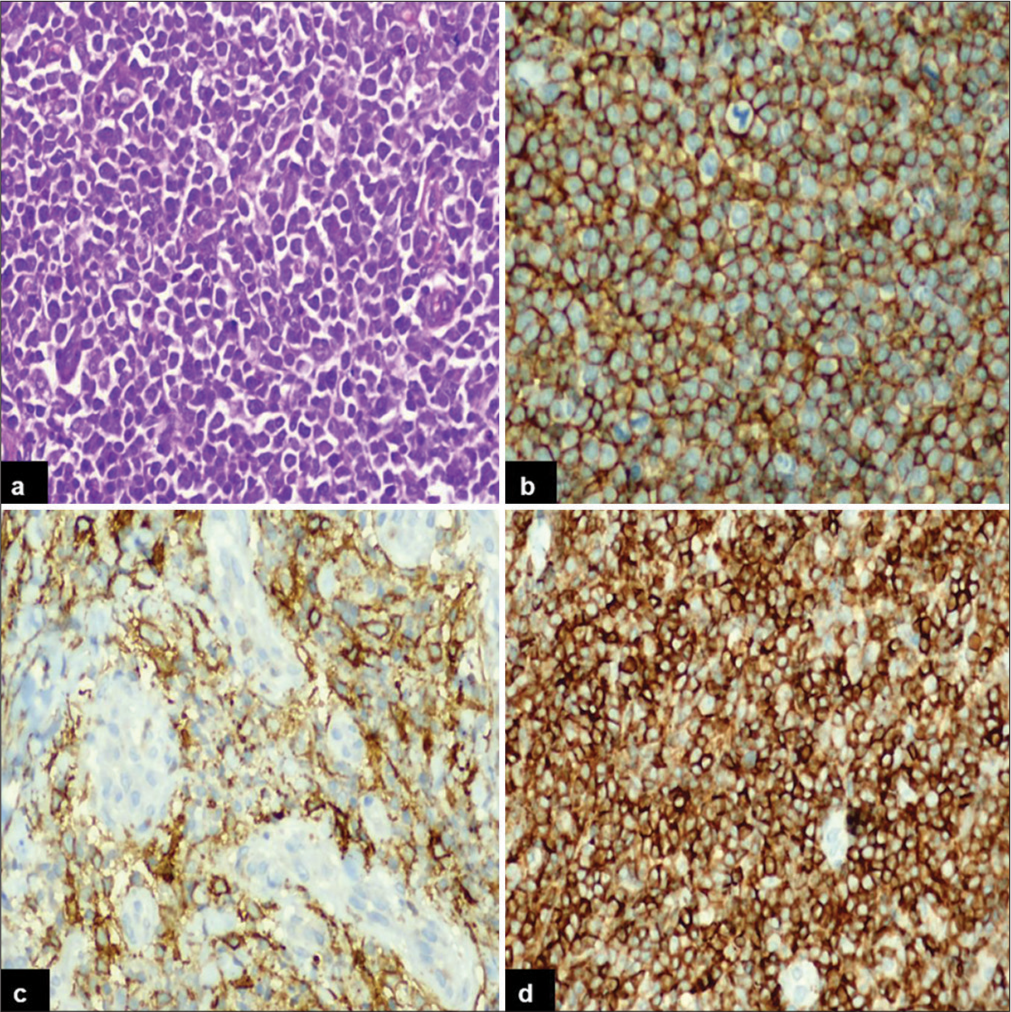
- Immunohistochemical findings of primary nodal-double-expressor phenotype of lymphoma, germinal center B-cell type: (a) Diffuse sheet of monomorphic large cells (Hematoxylin and eosin, ×40), (b) the tumor cells strongly express CD20, (c) CD10, and (d) BCL2 (Diaminobenzidine, ×40).
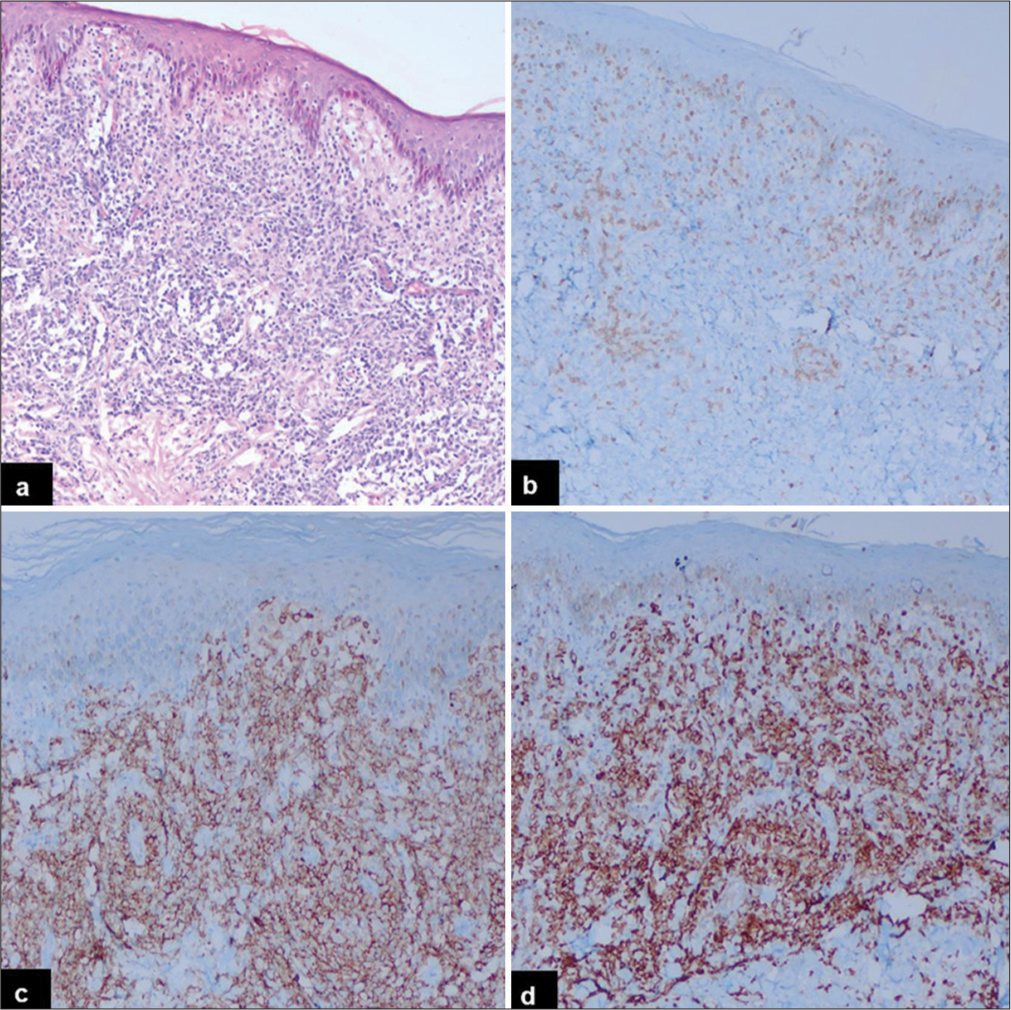
- Immunohistochemical findings of primary extranodal-double-expressor phenotype of lymphoma (skin), activated B-cell phenotype (low power): (a) A diffuse sheet of atypical lymphoid cells seen in the dermis (Hematoxylin and eosin, ×20). (b) CD3 highlights reactive T lymphocytes (Diaminobenzidine, ×20). (c) CD20 stains atypical B-cells (Diaminobenzidine, ×20). (d) The tumor cells express BCL2 (Diaminobenzidine, ×20).
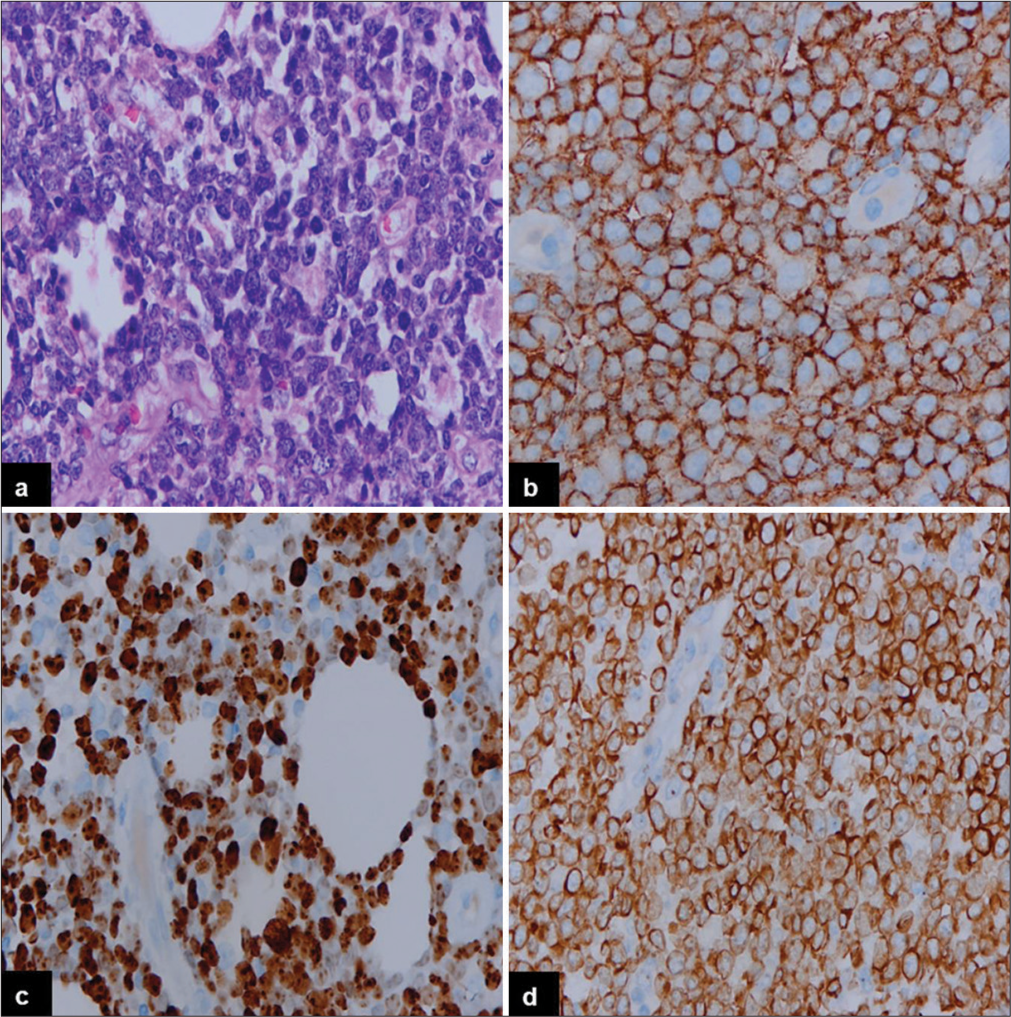
- Immunohistochemical findings of primary extranodal-double-expressor phenotype of lymphoma (skin), activated B-cell phenotype (high power): (a) The tumor cells are large in size with non-cleaved nuclei (Hematoxylin and eosin, ×40). (b) The tumor cells show immunopositivity for CD20, (c) MYC, (d), and BCL2 (Diaminobenzidine, ×40).
DISCUSSION
DLBCL is the most prevalent non-Hodgkin lymphoma, exhibiting variable clinical outcomes.[1,2] DEPL, characterized by concurrent expression of MYC and BCL2 proteins, represents a subtype with a poorer prognosis necessitating aggressive interventions.[9,10]
In our study, 28.7% of DLBCL cases were classified as DEPL, a frequency comparable to previous studies.[6,11] Among 167 cases of DLBCL, Johnson et al. showed 21% DEPL.[3] In a study by Hu et al., 34% of the cases displayed dual expression.[5] Ananthamurthy found 27.5% to be DEPL out of a total of 40 cases.[11] Overall, 20–30% of patients with DLBCL demonstrated coexpression except for the study by Mehta et al., which stated a lower frequency of 11.6%, and Hashmi et al., which documented a higher frequency of 35.8%.[9,12] Discrepancies in reported frequencies may be attributed to variations in MYC/BCL2 cutoffs in different studies.
We found that DEPL was more common in males, consistent with many previous reports, except for the findings by Hashmi et al. who reported females as a predominant population.[9,11,12] The comparable age at diagnosis (56 years) aligns with existing literature.[3,5,7] Most DEPL patients presented with adverse features such as B symptoms, elevated LDH levels, poor ECOG-PS, and higher-stage disease (III/IV) with BM involvement, in agreement with findings by Johnson et al. and Green et al.[3,4] ABC-type phenotype was more common in DEPL in our cohort. Hu et al. and Hashmi et al. reported similar findings and postulated that ABC phenotype in DEPL may pose an inferior prognosis.[5,12] However, the study by Ananthamurthy found almost equal representation of both GCB and ABC phenotypes in DEPL.[11] The association of coexpression of MYC/BCL2 with a higher Ki67 labeling index, as observed in our study, corroborates findings by Hashmi et al.[12] However, the role of the Ki67 labeling index in boosting DLBCL cases for dual expression or genomic studies remains debated, as highlighted by Mehta et al.[9]
A meticulous review of published literature revealed few studies documenting the occurrence and clinicopathological parameters of DEPL.[13,14] Only a handful of studies explored the clinicobiologic profiles of PN versus PEN-DLBCL patients, depicting significant clinical and genetic differences between them.[15-17] To the best of our knowledge, this study is the first attempt to explore the clinicopathological features of DEPL based on their primary sites.
The observed predominance of PN-DEPL over PENDEPL in our study, reflecting the higher frequency of PNDLBCL, aligns with existing literature.[18,19] This finding is in agreement with the study by Hashmi et al. but differs from Mehta et al., who reported an equal distribution of DEPL in nodal versus extranodal sites in the cohort of 20 cases.[9,12] A slight male predominance was noted in both PN-DEPL and PEN-DEPL groups in our study, consistent with studies by Shi et al., Candelaria et al., and Khan et al. concerning the PN/PEN-DLBCL cohort.[20-22]
Significant associations between PN-DEPL and PEN-DEPL were observed in B symptoms, LDH level, disease stage at presentation, BM involvement, pathological subtype, Ki67 index, and R-IPI. Despite the relative rarity of DEPL, we attempted to draw parallels between DLBCL and DEPL based on their primary sites. Møller et al. reported that patients with PEN-DLBCL were older and had higher ECOG-PS.[19] However, in our study, no significant association was found regarding age and ECOG-PS between PN-DEPL and PENDEPL patients.
PN-DEPL patients were more likely to present with B symptoms (82%), elevated LDH levels (73%), stage III/IV disease (71%), and maximum R-IPI score (64%) compared to PEN-DEPL patients, indicating a higher tumor burden. These findings align with studies by Shi et al.[20] and Khan et al.[22] Consistent with previous literature, we noticed that ABC-type phenotype (80%), Ann Arbor stage I/II (67%), Ki67 index >90% (93%), and BM involvement (87%) were more common in PEN-DEPL.[20] In contrast, Khan et al.[22] reported a lower rate of BM involvement in PEN-DLBCL, and Kim et al. found no differences in the frequencies of GCB and ABC subtypes in the compared groups.[18] Our observation, however, complies with the study by Candelaria et al.,[21] which also showcased the gastrointestinal tract as the most common site of extranodal lymphoma. The variation in findings among different studies emphasizes the complexity and heterogeneity of DEPL in different primary sites.
Numerous studies have aimed to assess the relative prognostic importance of genetic alterations and phenotypic and microenvironmental biomarkers in DLBCL. Although high-throughput proteomic profiling has not yet been applied to DLBCL subtyping, the concurrent expression of MYC and BCL2 proteins is a well-established prognostic marker in this lymphoma type.[23] So far, only a handful of studies have reported on the coexpression of MYC/BCL2 in different primary sites, and therefore, the prognostic role of double expression status in this clinical outline remains unpredictable. Future studies are recommended to evaluate the reasons and factors contributing to these unknown associations.
The study’s strength lies in the availability of a complete IHC workup, allowing an unambiguous diagnosis of DEPL and an examination of its characteristic profile based on primary sites. This study provides an estimate of the burden of this aggressive entity, encouraging further prognostic studies and therapeutic trials.
The drawback in our study involved the fact that DEPL cases subjected to molecular testing might have shown gene rearrangement (MYC/BCL2). Depending on the results, patients of DEPL would have stratified to a smaller cohort of DHL cases, thus altering the true sample size. It is also worth mentioning here that, unlike the reproducibility of the molecular cytogenetic technique, the IHC analysis has more variability associated with it. Another limitation can be the cutoff threshold assigned for consideration of positivity for MYC and BCL2 oncoprotein expression might have compromised the sample size and subsequent associations. Furthermore, we could not compare the outcomes of DEPL, PN-DEPL, and PEN-DEPL patients, which need to be urgently validated in a larger prospective cohort.
CONCLUSIONS
The retrospective nature of the study, resource constraints, and limitations related to the reproducibility of IHC analysis, molecular testing, and follow-up data should be acknowledged. Despite these limitations, the study comprehensively characterizes the clinical and pathological framework of DEPL, highlighting significant differences between PN-DEPL and PEN-DEPL. Testing for MYC and BCL2 rearrangements remains imperative in this subset to survey if they delineate to DHL. Further, research with larger prospective cohorts and long-term follow-up is recommended to assess molecular differences and understand the biological behavior of DEPL in nodal and extranodal primary sites for optimal treatment selection.
Ethical approval
The research/study was approved by the Institutional Review Board at Sri Aurobindo Medical College and Postgraduate Institute, number SAIMS/RC/IEC/156/23, dated July 10, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- WHO classification of tumours of haematopoietic and lymphoid tissues France: International Agency for Research on Cancer; 2017.
- [Google Scholar]
- The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia. 2022;36:1720-48.
- [CrossRef] [PubMed] [Google Scholar]
- Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460-7.
- [CrossRef] [PubMed] [Google Scholar]
- MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: A report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021-31.
- [CrossRef] [PubMed] [Google Scholar]
- MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165:382-91.
- [CrossRef] [PubMed] [Google Scholar]
- MYC or BCL2 copy number aberration is a strong predictor of outcome in patients with diffuse large B-cell lymphoma. Oncotarget. 2015;6:18374-88.
- [CrossRef] [PubMed] [Google Scholar]
- Double hit and double expressors in lymphoma: Definition and treatment. Cancer. 2018;124:4622-32.
- [CrossRef] [PubMed] [Google Scholar]
- Double hit and double expresser diffuse large B cell lymphoma subtypes: Discrete subtypes and major predictors of overall survival. Indian J Hematol Blood Transfus. 2020;36:627-34.
- [CrossRef] [PubMed] [Google Scholar]
- Double-hit and double-protein-expression lymphomas: Aggressive and refractory lymphomas. Lancet Oncol. 2015;16:e555-67.
- [CrossRef] [PubMed] [Google Scholar]
- An immunohistochemical study of double-expressor lymphomas and its correlation with cell of origin. J Cancer Res Ther. 2023;19:S0.
- [CrossRef] [PubMed] [Google Scholar]
- Double-expressor phenotype (BCL-2/cMYC Coexpression) of diffuse large B-Cell lymphoma and its clinicopathological correlation. Cureus. 2021;13:e13155.
- [CrossRef] [Google Scholar]
- Double or triple-expressor lymphomas: Prognostic impact of immunohistochemistry in patients with diffuse large B-cell lymphoma. Hematol Transfus Cell Ther. 2020;42:192-3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical impact of the cell-of-origin classification and the MYC/BCL2 dual expresser status in diffuse large B-Cell lymphoma treated within prospective clinical trials of the German high-grade non-Hodgkin's lymphoma study group. J Clin Oncol. 2017;35:2515-26.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and treatment response of double expression of MYC and BCL2 in patients with diffuse large B-cell lymphoma: A systematic review and meta-analysis. Cancers (Basel). 2021;13:3369.
- [CrossRef] [PubMed] [Google Scholar]
- The revised international prognostic index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857-61.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of double expression of C-MYC/BCL2 protein and cell of origin subtypes on the outcome among patients with diffuse large B-Cell lymphoma: A single Asian center experience. Asian Pac J Cancer Prev. 2018;19:1229-36.
- [Google Scholar]
- Biological characterization of nodal versus extranodal presentation of diffuse large B-Cell lymphoma using immunohistochemistry. Clin Lymphoma Myeloma Leuk. 2011;11:403-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse large B-cell lymphoma: Clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol. 2004;124:151-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: Analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res. 2019;31:152-61.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of primary extranodal versus nodal diffuse large B-cell lymphoma: A retrospective cohort study in a cancer center. Rev Invest Clin. 2019;71:349-58.
- [CrossRef] [PubMed] [Google Scholar]
- No difference in treatment outcome between patients with nodal versus extranodal diffuse large B-cell lymphoma. J Clin Transl Res. 2023;9:37-49.
- [Google Scholar]
- Genetic subtyping and phenotypic characterization of the immune microenvironment and MYC/BCL2 double expression reveal heterogeneity in diffuse large B-cell lymphoma. Clin Cancer Res. 2022;28:972-83.
- [CrossRef] [PubMed] [Google Scholar]






