Translate this page into:
Appraisal of six sigma in pre-analytical phase of clinical biochemistry laboratory
*Corresponding author: G. Parkavi, Department of Biochemistry, SRM Medical College Hospital and Research Centre, Chennai, Tamil Nadu, India. pg4141@srmist.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Parkavi G, Arul Senghor KA, Vinodhini VM. Appraisal of six sigma in pre-analytical phase of clinical biochemistry laboratory. J Lab Physicians. 2024;16:530-5. doi: 10.25259/JLP_163_2024
Abstract
Objectives:
The aim of the study is to measure the performance of pre-analytical phase of a clinical biochemistry laboratory using sigma metrics and the six sigma scale.
Materials and Methods:
The study included documented data of blood sample rejection from March 2023 to February 2024 and follow-up data from March 2024 to August 2024. International Federation of Clinical Chemistry and Laboratory Medicine developed Quality Indicators (QIs) used are QI-9 Wrong tubes; QI-10 Hemolyzed samples; QI-11 Clotted samples; QI-12 Insufficient samples; QI-14 Damaged samples in transport; and QI-15 Mislabeled samples. Based on “Six Sigma Quality Design and Control” established by Dr. Westgard, the sigma metric was calculated for the above-mentioned QIs.
Statistical analysis:
Obtained data were entered and analyzed using Microsoft Excel 2021.
Results:
Out of 162,380 received samples, 547 samples were rejected as not satisfied with the sample acceptance criteria. The most common pre-analytical error in the observed QIs is hemolyzed samples (458), followed by insufficient sample volume (55). The Sigma score of QI-10 was determined to be 4.81, whereas QI-9, QI-11, QI-12, and QI-15 were well maintained and graded excellent. Following training sessions, the follow-up month revealed a sigma score of 4.98 for QI-10.
Conclusions:
Six sigma metrics are a competent means to measure the performance of pre-analytical QIs in a clinical biochemistry laboratory. The observed QIs were effectively managed (>4 σ).
Keywords
Pre-analytical errors
Quality indicators
Sample rejection
Sigma score
Biochemistry
INTRODUCTION
Ensuring the quality of medical diagnostics is essential to achieve the goal of secure healthcare for patients. Among other clinical specialties, laboratory medicine is essential to ensure patient safety.[1] A clinical laboratory can be divided into three different phases: pre-analytical, analytical, and post-analytical. Test selection, patient identification, sample collection, sample handling, sorting, pipetting, and centrifugation are all included in the pre-analytical step.[2]
Inaccurate protocols attributed to the pre-analytical stage could arise from negligence in carefully carrying out the standard procedures. The pre-analytical stage is recognized as one of the most challenging aspects of testing for laboratory personnel and is also the most vulnerable part of the entire process.[3]
To evaluate and improve the testing process, the International Federation of Clinical Chemistry developed Quality Indicators (QIs), which quantify healthcare quality across multiple aspects and compare it to predetermined criteria.[4] Sigma metrics are useful in identifying process improvement opportunities. Defects per million opportunities (DPMO) is a metric used to quantify the performance of QIs.
DPMO = (Number of errors × 1000,000)/Total number of specimens
DPMO is converted into sigma metrics. Process quality is evaluated using the sigma scale, where 3σ is the lowest performance that can be tolerated, and 6σ is world-class quality.[5]
Missing patient identification, using the wrong container, and samples not satisfying the acceptance criteria are the most common pre-analytical errors.[6]
Although there are international criteria for sample collection and standardization, there is a notable lack of adherence to these norms, especially when sampling is done by nurses or young doctors without the assistance of laboratory personnel.[7] There is much potential for improvement in the pre-analytical stage of laboratory medicine over the long journey toward guaranteeing patient safety.[8]
Locally, there is little information available about the documentation, root cause analysis, and preventive measures used in the occurrence of laboratory errors.[9] The range of the reported error rate for the complete laboratory testing procedure is 0.1–9.3%.[10] Pre-analytical errors can be reduced with the use of strict quality assurance processes and effective staff training.[11] Less than 10% are analytical errors, which were extensively studied in prior studies; pre-analytical errors, on the other hand, are said to be responsible for 46–68.2% of laboratory errors.[12]
Our study seeks to assess the primary reasons behind pre-analytical errors and quantify the performance of the pre-analytical phase of a clinical biochemistry laboratory of a tertiary care hospital using sigma metrics and the Six Sigma scale.
MATERIALS AND METHODS
This study was conducted at the Central Biochemistry Laboratory of the Hospital. A phase-wise systematic approach was planned. Phase I (Before training) internal audit was conducted in the phlebotomy area, and the gap analysis report was reviewed by the technical supervisor. The cause-and-effect tool was used to identify and analyze the root causes of sample rejections and repeat samples. The following challenges were identified: improper collection technique (prolonged tourniquet), reduced use of vacutainer needles, improper mixing of samples, and not following the order of draw. Usually, the QIs are monitored at the pre-analytical, analytical, and post-analytical phases of sample processing on a daily basis.
As per ISO/DIS 22367:2019 Application of Risk Management to Medical Laboratories, Table 1 pre-analytical QIs were monitored.
| QI score | Type of rejection |
|---|---|
| QI-9 | Wrong tube |
| QI-10 | Hemolyzed |
| QI-11 | Clotted sample |
| QI-12 | Insufficient sample |
| QI-14 | Damaged in transport |
| QI-15 | Mislabeled tubes/samples |
QI: Quality indicator
The study included documented data of pre-analytical QIs addressing blood sample rejection from March 2023 to February 2024 and follow-up data from March 2024 to August 2024. The outpatient and inpatient blood samples were collected by phlebotomy staff, ward staff, and doctors.
Samples received at the sample reception area are checked based on sample acceptance; if not, samples were rejected based on QIs, and the monthly reviewed data were recorded.
Phase II Calculation of DPMO for the above QIs with the formula:
DPMO = (Number of errors × 10,00,000)/Total number of specimens
Based on “Six Sigma Quality Design and Control” established by Dr.Westgard, the sigma metric was calculated for the above-mentioned QIs (Sigma = [Total allowable error-bias]/Coefficient of variation).[13] The sigma levels of the QIs determine the performance of those processes. Table 2 shows the sigma process levels 1–6.[13]
| Sigma | DPMO | Defect-free (%) |
|---|---|---|
| 1 | 691,462 | 30.85 |
| 2 | 308,538 | 69.146 |
| 3 | 66,807 | 93.319 |
| 4 | 6210 | 99.379 |
| 5 | 233 | 99.977 |
| 6 | 3.4 | 99.99966 |
DPMO: Defects per million opportunities
Phase III (Training session) weekly once sample collection training program was conducted using vacutainer needles with safety lok by the internal technical supervisor and an external expert. The sample collection procedure was assessed using a competency assessment tool; if needed, repeated training sessions were conducted. Phase IV Quality improvement strategies such as the use of safety-engineered needles, proper sample collection procedures, and a new staff induction program were followed on a regular basis.
RESULTS
This study included the clinical biochemistry samples received at the central laboratory during the study period of March 2023 to February 2024, and follow-up data from March 2024 to August 2024 were reviewed monthly. QIs used in this study for rejection of samples are QI-9, 10, 11, 12, 14, and 15. Out of 162,380 received samples, 547 samples were rejected based on the rejection criteria of QIs. Figure 1 shows the pie chart depicting the rejection percentage of each QI. The most common pre-analytical error in the observed QIs is QI-10 hemolyzed samples (458), followed by insufficient sample (QI-12), wrong tubes (QI-9), clotted samples (QI-11), and mislabeled samples (QI-15) in order. All the samples were transported to the central laboratory in biohazard-labeled containers. Thereby, there was no sample loss due to damage while transporting (QI-14). Figures 2 and 3 depict the bar chart of the number of rejections during the study period and follow-up period, respectively.
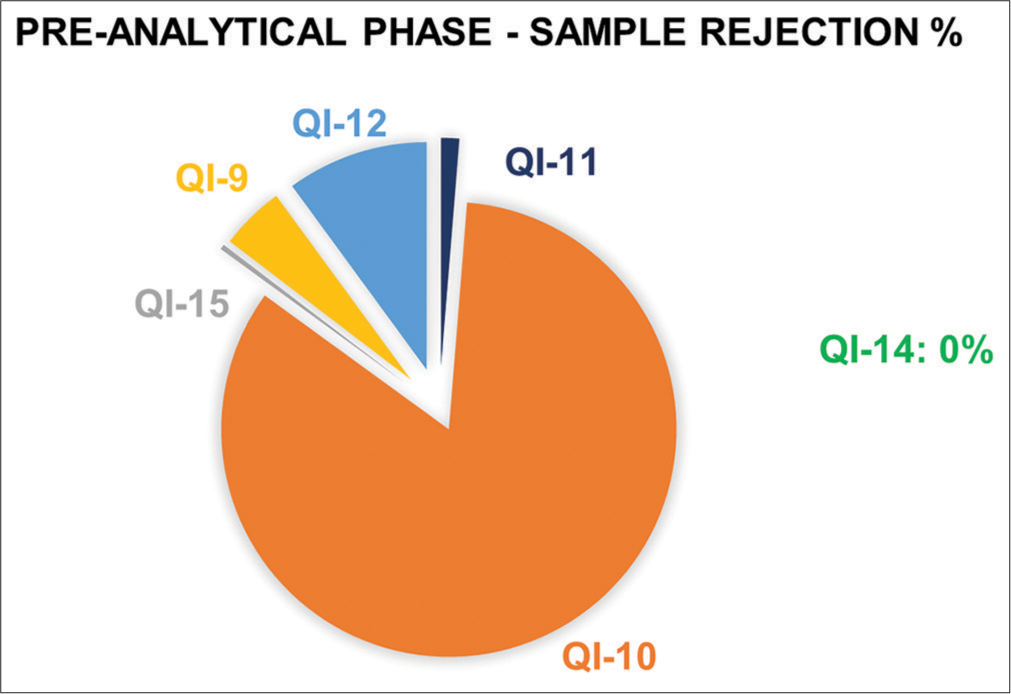
- Pie chart depicting the rejection percentage of each quality indicator (QI-9 Wrong tubes, QI-10 Hemolyzed samples, QI-11 Clotted samples, QI-12 Insufficient samples, QI-14 Damaged samples in transport, and QI-15 Mislabeled samples). QI: Quality indicator.
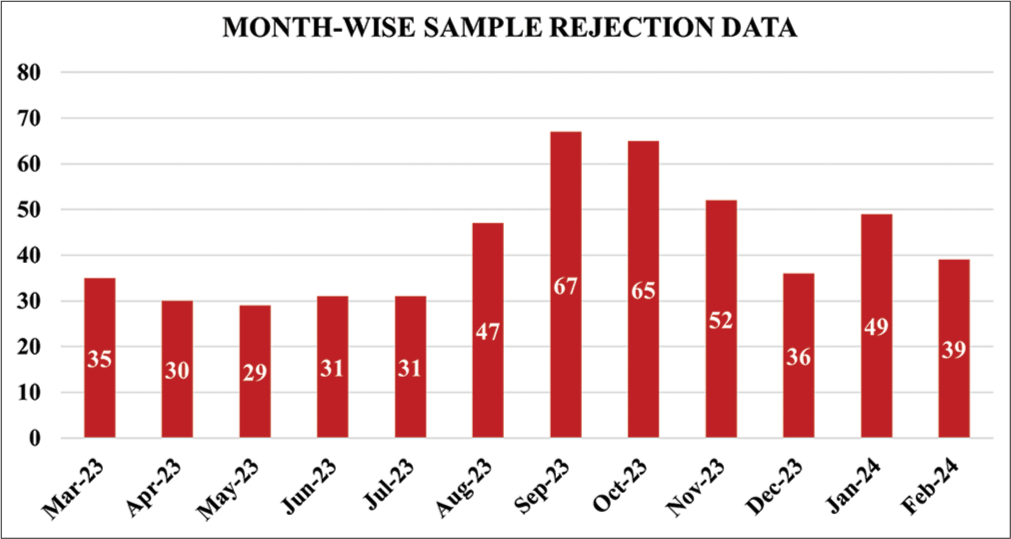
- Bar diagram showing the sample rejections during the study period.
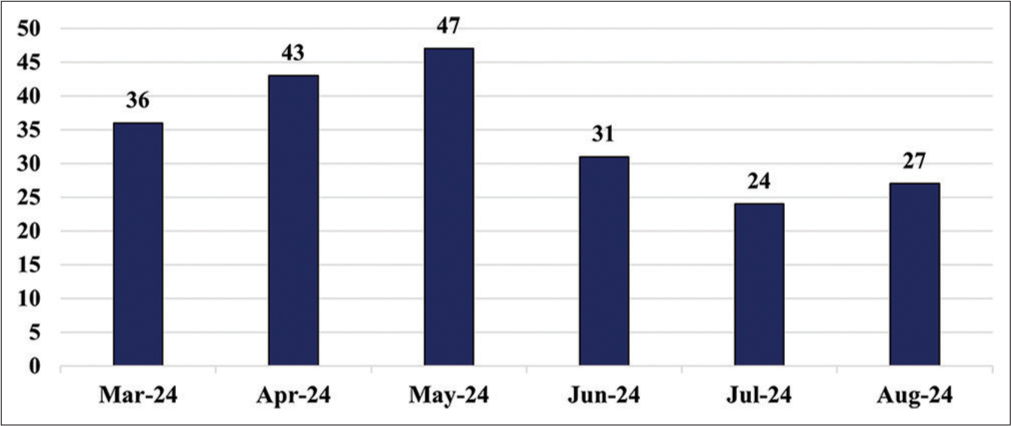
- Bar diagram showing the sample rejections during the follow-up period.
In the months of September and October 2023, the workload was more with increased patient statistics. Moreover, there was an inadequate workforce and increased work pressure for the existing staff that resulted in the high sample rejections predominantly due to hemolysis QI-10.
Table 3 shows the sigma metrics of each QI analyzed in the pre-analytical phase. The Sigma score of QI-10 was analyzed to be 4.81, whereas QI-9, 11, 12, and 15 were all well-maintained and graded excellent. Stringent protocols were practiced during the follow-up period (March 2024–August 2024), which showed improvements and revealed a better Sigma score of 4.98 for QI-10. Figure 4 shows the trend of sample rejections due to hemolysis (QI-10) in which the follow-up month displays better progress.
| QI score | Type of rejection | Rejection% | Sigma | Outcome |
|---|---|---|---|---|
| QI-9 | Wrong tube | 4.57 | 5.55 | Excellent |
| QI-10 | Hemolyzed | 83.7 | 4.81 | Good |
| QI-11 | Clotted | 1.27 | 5.84 | Excellent |
| QI-12 | Insufficient sample | 10.05 | 5.36 | Excellent |
| QI-14 | Damaged in transport | 0 | - | - |
| QI-15 | Mislabeled | 0.3 | 6 | Gold standard |
QI: Quality indicator
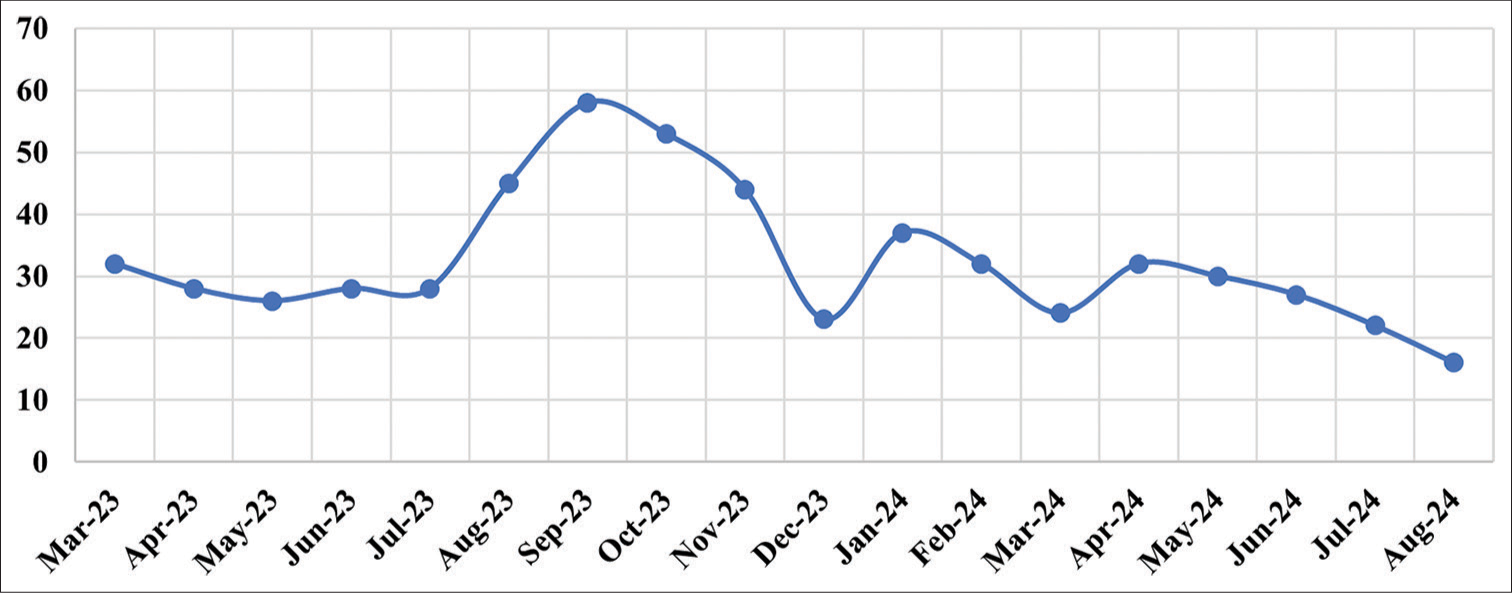
- Trend of sample rejections due to hemolysis (QI-10) in which the follow-up period displays better progress. QI: Quality indicator.
DISCUSSION
The accuracy and reliability of test results rely on the quality of the received specimens. Basic protocols are ensured for collecting the right sample on the right patient at the right time with appropriate patient preparation. In clinical laboratories, pre-analytical errors are the most common cause of specimen rejection. Rejected specimens cause despair and difficulty during repeat collection, which affects the turnaround time of test results. For this reason, samples are subjected to satisfy the specimen acceptance criteria, which is very much vital in clinical laboratories’ quality control processes.[14]
As per our documented data, it was revealed that for every 1000 investigations, 3.36 samples were rejected overall. Furthermore, as notified in the aspects of standard QIs, the rejections were represented as sigma metric values established by Westgard.[13]
The foremost reason for sample rejection is hemolysis, which is the most common pre-analytical error observed in our diagnostic center, followed by insufficient samples. Pre-analytical errors are characterized as human-dependent and controllable sources of errors that occur between the time a doctor orders a laboratory test and the sample has been prepared for analysis.[15]
As observed in the frequent occurrence of hemolyzed samples, immediate corrective action of repeat samples is requested, and reanalysis was carried out in view of patient care. As corrective action, root cause analysis was carried to identify the source of error [Figure 5]. It was revealed that ward staff or trainees need to master the art of venipuncture using flashback needles. Only repeated training and retraining make a person competent. Subsequently, phlebotomists, nurses, technicians, medical interns, and residents were trained in the fundamentals of collecting blood samples, as well as potential causes of error and optimal methods to mitigate them.[16]
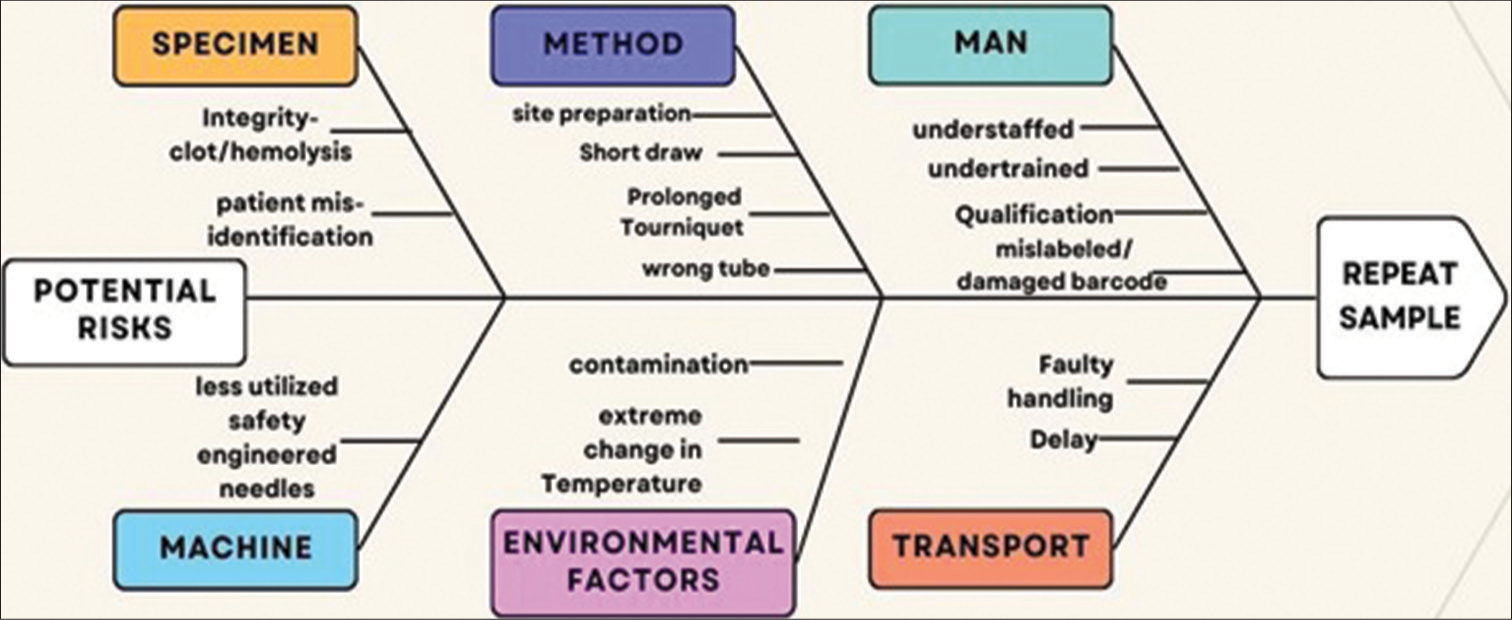
- Fishbone diagram analysis of root causes of sample rejection.
Figure 6 displays the training session conducted for critical care unit ward staff. Skill-based competency assessment was carried out for the phlebotomist in blood sample collection using vacutainer needles and tubes.

- Training session conducted for critical care unit ward staff. Skill-based competency assessment was carried out for the phlebotomist in blood sample collection using vacutainer needles and tubes.
Post-training: The number of sample rejections was drastically reduced at high-risk areas such as the outpatient department, Intensive Care Unit, and Casualty, where the samples were collected by trained staff. According to Clinical and Laboratory Standards Institute (CLSI) guidelines, avoidable laboratory errors negatively impacted patient outcomes around 24.4% of the time, resulting in repeat testing, longer hospital stays, and higher expenses.[17]
Therefore, to establish procedures in effect for identifying and managing pre-analytical variables, a quality manual for pre-analytical variables is required. This constitutes an essential element of laboratory quality that must not be hampered by methods of analysis and quality control procedures. According to CLSI guidelines, both patient preparation and sample acceptance criteria should be mentioned in the pre-analytical quality manual.[18]
The laboratory manual addresses the pre-analytical steps of sample collection, transportation, acknowledgment, and sample processing guidelines. All laboratory technicians should be aware of the ISO15189:2022 guidelines, in addition to international standards like ISO 6710, Good Clinical and Laboratory Practices, and the CSLI.[19] Newly joined phlebotomists and ward staff conducted orientation programs on guidelines and procedures of proper venipuncture technique, color-coded vacutainer tubes, and awareness on prevention of pre-analytical errors. The overall sigma score of the observed pre-analytical QIs before and after training sessions are tabulated in Table 4.
| Period | Total no. of rejections | Total no. of samples | Overall sigma score |
|---|---|---|---|
| March 2023–February 2023 (study period) | 547 | 162380 | 4.75 |
| March 2024–August 2024 (follow-up data) | 208 | 82642 | 4.84 |
The study implemented Six Sigma in the phlebotomy section, which was a data-driven methodology to identify defects, reduce errors, and facilitate enhanced patient care.
CONCLUSIONS
Six sigma metrics is a competent means to measure the performance of pre-analytical QIs in a clinical biochemistry laboratory. The observed QIs were effectively managed (>4 σ).
Clinical laboratories consistently face challenges due to specimen rejections for a variety of reasons, which must be treated seriously due to their potential to complicate the test process and adversely affect patient safety.
Medical laboratory professionals’ knowledge of pre-analytical errors and managing them may assist in lowering test result errors, which will eventually enhance the standard of care to patients.
Acknowledgments
The authors acknowledge the biochemistry laboratory technicians for their dedicated documentation of pre-analytical errors on a routine basis.
Ethical approval
The research/study was approved by the Institutional Review Board at the Ethics Committee of SRM Medical College Hospital and Research Center, SRM IST – Kattankulathur, number SRMIEC-ST0224-951, dated 03rd May 2024.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Challenges in preanalytical phase of laboratory medicine: Rate of blood sample nonconformity in a tertiary care hospital. EJIFCC. 2020;31:21-7.
- [Google Scholar]
- Estimating the intra-and inter-individual imprecision of manual pipetting. Clin Chem Lab Med. 2017;55:962-6.
- [CrossRef] [PubMed] [Google Scholar]
- The preanalytical phase-from an instrument-centred to a patient-centred laboratory medicine. Clin Chem Lab Med. 2023;61:732-40.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of quality indicators in pre-analytical phase of testing in clinical biochemistry laboratory of a tertiary care hospital in India. Int J Clin Biochem Res. 2020;7:354-6.
- [CrossRef] [Google Scholar]
- Preanalytical errors: A major issue in medical laboratory. Acta Sci Med Sci. 2019;3:93-5.
- [Google Scholar]
- Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin Biochem. 2017;50:568-73.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of blood sample rejection in a clinical laboratory of an oncology institute. Malays J Med Health Sci. 2021;17:49-54.
- [Google Scholar]
- Preanalytical nonconformity management regarding primary tube mixing in Brazil. J Med Biochem. 2017;36:39-43.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and types of preanalytical error in hematology laboratory of a tertiary care hospital in South India. J Lab Phys. 2018;10:237-40.
- [CrossRef] [PubMed] [Google Scholar]
- The results of a close follow-up of trainees to gain a good blood collection practice. J Med Biochem. 2020;39:355.
- [CrossRef] [PubMed] [Google Scholar]
- Finish before the start: Analyzing preanalytical sample errors in a tertiary care hematology laboratory. Indian J Pathol Microbiol. 2020;63:435-40.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting rejection: Analysis of specimen acceptability and rejection, and framework for identifying interventions in a single tertiary healthcare facility. J Clin Lab Anal. 2016;31:e22060.
- [CrossRef] [PubMed] [Google Scholar]
- Six Sigma quality design and control: Desirable precision and requisite QC for laboratory measurement processes In: Six Sigma calculators - Westgard. Madison, WI: Westgard QC; 2000.
- [Google Scholar]
- Quantification of pre-analytical quality indicators in a clinical laboratory and formulating the lean six sigma DMAIC strategy. J Indian Med Assoc. 2022;120:17-22.
- [Google Scholar]
- Factors affecting quality of laboratory result during ordering, handling, and testing of the patient's specimen at Hawassa University College of Medicine and Health Science comprehensive specialized hospital. J Multidiscip Healthc. 2020;13:809-21.
- [CrossRef] [PubMed] [Google Scholar]
- Procedures for the collection of diagnostic blood specimens by venipuncture; approved standard In: CLSI document H3-A6 (6th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
- [Google Scholar]
- Collection of diagnostic venous blood specimens In: CLSI standard GP41 (7th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
- [Google Scholar]
- Adopting role playfor training medical interns about sample collection and processing for laboratory investigations. Indian J Med Biochem. 2015;19:24-7.
- [Google Scholar]
- Detecting preanalytical errors using quality indicators in a hematology laboratory. Qual Manag Healthc. 2022;31:176-83.
- [CrossRef] [PubMed] [Google Scholar]






