Translate this page into:
Desmoplastic small round cell tumor of ileum presenting as polyps: A rare presentation
*Corresponding author: Sreerekha Jinkala, Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. sree.path177@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thambiraj P, Jinkala S, Jain A. Desmoplastic small round cell tumor of ileum presenting as polyps: A rare presentation in an adolescent male. J Lab Physicians. 2024;16:575-7. doi: 10.25259/JLP_54_2024
Abstract
Desmoplastic small round cell tumor (DSRCT) is a rare, highly aggressive, malignant tumor of undetermined histogenesis with a poor prognosis. Adolescent males are primarily affected with a typically abdominopelvic mass. Diagnosis is usually based on histologic analysis of biopsy and cytogenetic studies. Here, we report a 26-year-old adolescent male who presented with acute intestinal obstruction and was found to have multiple polypoidal masses in the small intestine. Histopathology showed small round cells arising from serosa infiltrating the submucosa and muscularis propria. Immunohistochemistry findings revealed positive staining for epithelial, mesenchymal, and neural markers, which confirmed the diagnosis of DSRCT. We report this rare presentation of DSRTC of the ileum with multiple polypoidal masses.
Keywords
Desmoplastic small round cell tumor
Ileum
Adolescent male
INTRODUCTION
Desmoplastic small round cell tumor (DSRTC) is a rare and extremely aggressive malignant mesenchymal tumor with an unclear histogenesis. It typically manifests with peritoneal involvement and an abdominal mass.[1] Gerald and Rosai first described this new entity in 1989.[2] With a median survival range of 17–25 months, DSRCT has an extremely poor prognosis. DSRCT primarily affects young adolescent males in their second and third decades, with a 4:1 male-to-female ratio. It is more common in these populations.[3] We present the case of a adult male who had multiple polypoidal masses in his small intestine in addition to acute intestinal obstruction.
CASE REPORT
A 26-year-old male presented with a right iliac fossa mass and acute intestinal obstruction. He had no remarkable family or medical history. Contrast-enhanced computed tomography showed multiple heterogeneously enhancing exophytic mass lesions (largest size ~16.1 cm in length, 10.5 cm in thickness) arising from the ileum, causing luminal narrowing and mild proximal bowel-loop dilation [Figure 1a]. Extra serosal spread involving hepatic flexure, ascending colon with asymmetric wall thickening reaching until cecum was noted. Loss of fat plane and focal infiltration of adjacent right lobe liver parenchyma was also seen. Multiple enlarged mesenteric, pelvic, and omental deposits were noted. Radiological differential diagnosis included gastrointestinal stromal tumor (GIST), lymphoma, and adenocarcinoma. The ileal segment was resected. Grossly, the ileal segment showed multiple polypoidal masses seen in the mucosal aspect, which on the cut surface appeared solid, gray-white, and homogenous [Figure 1b].
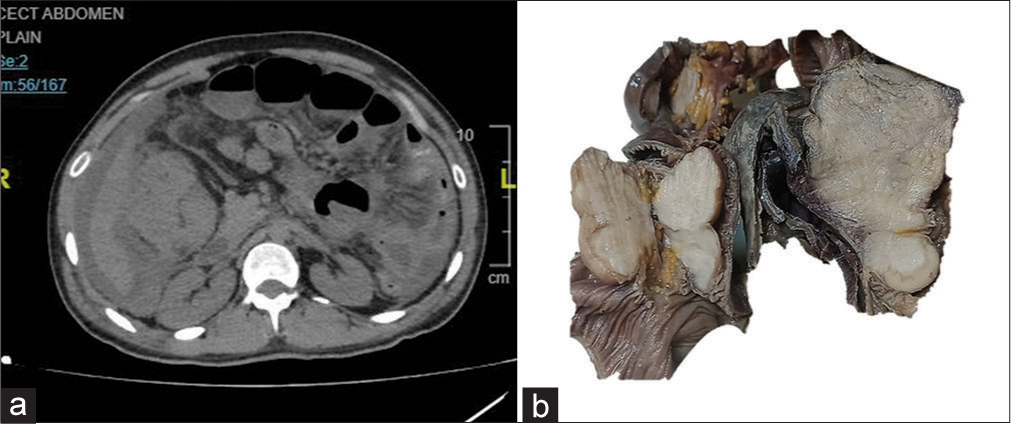
- (a) Contrast-enhanced computed tomography shows multiple exophytic masses in the serosal aspect of the intestine, causing luminal narrowing. (b) A gross image of multiple submucosal solid masses in the small intestine.
Histopathology showed six polypoidal submucosal lesions; all the lesions showed similar morphology. Polypoidal masses on microscopy showed small round cells arranged in sheets, cords, nests, and trabeculae arising from serosa. It infiltrates submucosa and muscularis propria. Extensive desmoplasia in subserosal connective tissue is seen separating the tumor nests [Figure 2a-c]. On immunohistochemistry (IHC), tumor cells were diffusely positive for desmin, CD99, neuron-specific enolase (NSE), and faintly for Cytokeratin [Figure 2d and e]. Tumor cells were negative for myogenic differentiation 1 (MyoD1) leukocyte common antigen, glial fibrillary acidic protein, and synaptophysin. CD117 and DOG-1 were negative, which ruled out GIST. The final diagnosis of a desmoplastic small round tumor (DSRCT) with multicentric involvement of the ileum was made. The patient succumbed to post-operative complications.
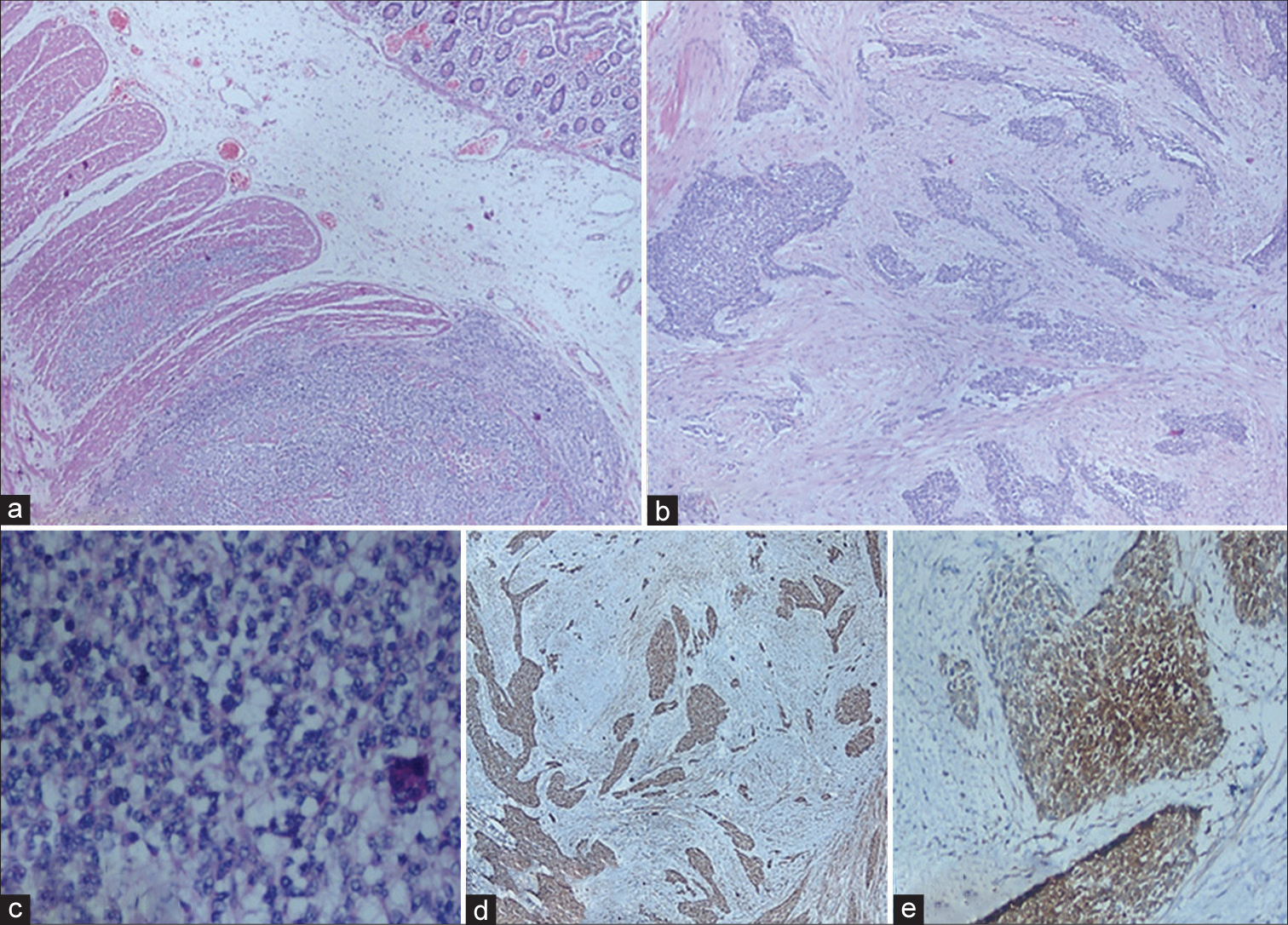
- (a) Submucosal tumor infiltrating muscularis propria with adjacent normal intestinal mucosa, ×100 H&E; (b) nests and cords of tumor cells with desmoplastic stroma, ×200, H&E; (c) sheets of small round cells with hyperchromatic nuclei with scant cytoplasm, ×400, H&E; and (d) desmin immunostain shows cytoplasmic positivity ×400, diaminobenzidine (DAB); (e) Neuron-specific enolase immunostain shows cytoplasmic positivity ×400, DAB. H&E: Hematoxylin and Eosin, DAB: Diaminobenzidine
DISCUSSION
DSRCT is typically associated with multiple peritoneal implants and occurs in the abdominal or pelvic cavity. The histogenesis of DSRCT is unknown as most of them arise from the peritoneal cavity without a primary visceral site of origin; they may arise from the mesothelium or submesothelial or subserosal mesenchyme.[4] Rare sites of DSRTC include the head and neck, base of the skull, para testicular region, ovary, brain, or thoracic viscera. The sites of extraperitoneal organ involvement can be seen in the lungs, liver, kidney, stomach, and pancreas.[5] The liver and lungs are the two most frequently affected sites of distant metastasis.[5] Ascites, weight loss, vomiting, nausea, and abdominal pain or distention are just a few of the non-specific clinical symptoms of DSRCT. On physical examination, a palpable mass in the pelvic or abdominal cavity may be found, and there may be multiple lesions of differing sizes.
A biopsy is usually recommended to diagnose this entity. Typically, cytogenetics and IHC markers are used to confirm DSRCT in its entirety. On light microscopy, it shows a small round cell morphology with extensive desmoplastic stroma. Typically, DSRCT expresses neural (CD56 and NSE), mesenchymal (vimentin), myogenic (desmin), and epithelial (cytokeratin and epithelial membrane antigen) markers. They test positive for Wilms tumor (WT)-1, a polyclonal antibody that targets the WT-1 protein’s amino terminus. DSRCT is characterized by the fusion of the Ewing sarcoma (EWS) gene’s N terminus to the WT-1 gene’s C terminus, which is a t(11;22) (p13;q12) chromosomal translocation.[6] A conclusive DSRCT diagnosis is provided by the existence of this translocation.
Other entities which can present as polypoidal masses in the small intestine are hamartomatous polyps and adenomatous polyps. Other tumors such as carcinoid tumors, GIST, and Non-Hodgkin lymphoma can also present as polypoidal masses. Other small round cell tumors such as lymphoma/leukemia, rhabdomyosarcoma, neuroblastoma, small cell mesothelioma, Ewings sarcoma and Wilms tumor should be kept in the differential diagnosis based on the location of the lesion due to the round cell morphology. However, these can be differentiated based on the site of the lesion, histomorphology, and specific IHC profile. DSRCT is recognized as undifferentiated sarcoma. A relevant panel of IHC markers helps in differentiating the other entities.
The treatment protocol of this case remains unclear due to the rarity of this lesion. However, surgical debulking surgery, with a 90% reduction of tumor mass, helps in better survival of the patient. Gross tumor resection has been found to have a 3-year survival rate of 58% overall, compared to 0% in incurable cases.[7] The effects of radiation therapy are not well studied.
CONCLUSIONS
DSRCT is a malignant tumor that is extremely aggressive and has a very poor prognosis. Presentation within the ileal wall with multiple polypoidal masses is what makes this case report interesting.
Author contributions
PT: Manuscript writing; SJ: Diagnosis, manuscript editing and revision; AJ: Manuscript editing and revision.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Desmoplastic small round cell tumor: Current management and recent findings. Sarcoma. 2012;2012:714986.
- [CrossRef] [PubMed] [Google Scholar]
- Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177-83.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22) (p13;q12): Desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998;16:3028-36.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-abdominal desmoplastic small round cell tumor: Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499-513.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis and management of desmoplastic small round cell tumor: A review. Curr Opin Oncol. 2011;23:385-9.
- [CrossRef] [PubMed] [Google Scholar]
- WT1 staining reliably differentiates desmoplastic small round cell tumor from Ewing sarcoma/primitive neuroectodermal tumor: An immunohistochemical and molecular diagnostic study. Am J Clin Pathol. 2000;114:345-53.
- [CrossRef] [PubMed] [Google Scholar]
- Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg. 2005;40:251-5.
- [CrossRef] [PubMed] [Google Scholar]






