Translate this page into:
Observation of matrix metalloproteinase-9 levels in patients with neurocysticercosis at a tertiary care super specialty institute in North India
*Corresponding author: Manodeep Sen, Department of Microbiology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. sen_manodeep6@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Raj N, Yadav M, Sen M, Das A, Agarwal J, Singh AK. Observation of matrix metalloproteinase-9 levels in patients with neurocysticercosis at a tertiary care super specialty institute in North India. J Lab Physicians. 2025;17:81-7. doi: 10.25259/JLP_206_2024
Abstract
Objectives
Elevated levels of matrix metalloproteinase-9 (MMP-9) in both serum and cerebrospinal fluid have been observed in patients diagnosed with cysticercus granuloma. These elevated levels significantly contribute to the onset of symptoms, such as active seizures, in patients with neurocysticercosis (NCC). This study aimed to compare and correlate MMP-9 levels between NCC cases and control subjects.
Materials and Methods
In this prospective case-control study, 45 symptomatic cases of NCC and 30 controls were enrolled. Serum MMP-9 levels were measured using a commercially procured enzyme-linked immunosorbent assay kit (QayeeBio, Shanghai, China) in all collected serum samples, following the manufacturer’s instructions.
Statistical analysis
Data were presented as mean ± standard deviation (SD), frequencies, and percentages. Chi-square and unpaired t-tests were used for comparisons, while receiver operating curve analysis was performed to assess diagnostic accuracy metrics, with a significance threshold of P < 0.05.
Results
The mean age of cases and controls was 31.20 ± 11.98 years and 55.40 ± 9.80 years, respectively. The mean ± SD values of MMP-9 were significantly higher in NCC cases (13.42 ± 5.70 ng/mL) compared to controls (8.49 ± 4.21 ng/mL) (P < 0.00001). At a cutoff of >10 ng/mL, serum MMP-9 levels correctly identified 73.3% of the 45 confirmed NCC cases (33/45; sensitivity) and correctly excluded 63.3% of the 30 controls (19/30; specificity). This threshold resulted in a positive predictive value of 75.0% and a negative predictive value of 61.3%.
Conclusions
MMP-9 demonstrated the ability to distinguish NCC cases from controls with a sensitivity of >73% and may serve as a marker for predicting and diagnosing symptomatic NCC. However, due to its moderate sensitivity and specificity, MMP-9 should not be used as a standalone diagnostic marker. Instead, it may complement existing serological and imaging tools to diagnose NCC.
Keywords
Enzyme-linked immunosorbent assay
Metalloproteinases-9
Neurocysticercosis
Seizure
Taenia solium
INTRODUCTION
Neurocysticercosis (NCC) is a central nervous system (CNS) infection caused by the larval stage of the tapeworm Taenia solium.[1] It is the most prevalent infectious cause of acquired epilepsy in developing countries and occurs when parasite eggs are ingested.[2] According to the World Health Organization, an estimated 2.5–8.3 million cases of NCC occur annually, with a disability-adjusted life year burden of 2.8 million.[3] NCC has a diverse clinical presentation and is a slowly progressive disease, most commonly manifesting as active seizures.[4] The severity of symptoms is influenced by the extent of inflammation in the host brain.[5]
With advancements in imaging techniques and increased accuracy of serological testing, the diagnosis of NCC has significantly improved over the past two decades. In most cases, an accurate diagnosis can be achieved through a combination of immunodiagnostic and radiological tests. NCC lesions can be correctly recognized using computed tomography (CT) and magnetic resonance imaging (MRI). However, differentiating NCC from other brain lesions, such as gliomas, tuberculomas, and hydatid cysts, can present diagnostic challenges.[6]
The most effective serological test currently available for the diagnosis of NCC is the enzyme-linked immunoelectrotransfer blot (EITB) assay, which has a sensitivity of 98% and a specificity of 100%.[7] The EITB assay does not confirm the presence of an active infection; rather, it detects antibodies against the larval antigen. A positive result may occur in individuals previously exposed to the parasite antigen, those who have already undergone treatment, or those with cysts located outside the CNS.[8] Alternative methods for detecting T. solium antibodies, such as the enzyme-linked immunosorbent assay (ELISA), which uses crude or refined parasite antigen lysates with immunoglobulin G (IgG) as the target immunoglobulin, generally have lower specificity and sensitivity compared to the EITB assay.[9] Antigen detection aids in diagnosing NCC, as the antigen ELISA (Ag-ELISA) detects larval antigens in the bloodstream, making it more likely to identify individuals with an active infection. AgELISA, however, cannot be used for diagnosing calcific lesions and has a low sensitivity, ranging from 72% to 86%.[8]
The multi-domain endopeptidases known as matrix metalloproteinases (MMPs) are produced by inflammatory cells, including neutrophils, astrocytes, microglia, and monocytes.[10] MMP-9 is a zinc-dependent enzyme involved in extracellular matrix degradation and blood-brain barrier (BBB) disruption, playing a significant role in the pathogenesis and progression of various neurological infections, including bacterial, viral, and parasitic diseases.[11]
In bacterial meningitis and tuberculous meningitis, elevated MMP-9 levels contribute to BBB permeability, inflammatory cell infiltration, and neuronal damage.[12,13] Similarly, in viral infections such as human immunodeficiency virus-associated neurological disorders, MMP-9 dysregulation exacerbates neuroinflammation and disease progression.[14] In parasitic infections, MMP-9 plays a detrimental role in NCC by increasing BBB permeability and inflammation; in Toxoplasma gondii infection by facilitating leukocyte migration into the CNS; in Acanthamoeba infections by altering MMP-9/tissue inhibitor of metalloproteinases balance leading to neuronal damage and in leishmaniasis by enabling CNS infiltration of parasites and immune cells.[15-18]
Studies have demonstrated that levels of MMP-9 in serum and cerebrospinal fluid (CSF) are significantly elevated in symptomatic NCC patients and play a crucial role in the development of symptoms, such as active seizures.[19] MMP-9 can be used as a potential marker for NCC due to its critical role in inflammation, tissue remodeling, and the breakdown of the extracellular matrix, processes that are highly relevant in the pathogenesis of NCC.[10] With this background, this study was conducted to estimate, compare, and correlate MMP-9 levels in NCC cases and control.
MATERIALS AND METHODS
This was a one-year (September 2020–September 2021) prospective case-control research study conducted in the Neurology and Microbiology departments of the Dr. Ram Manohar Lohia Institute of Medical Sciences in Lucknow. The study was approved by the Dr. Ram Manohar Lohia Institute of Medical Sciences (Dr. RMLIMS), Lucknow Institutional Ethics Committee, as per the approval letter reference number RMLIMS/IEC/74/18 dated January 2, 2019.
For a prevalence of 13% with a 90% confidence level and a 10% margin of error, a sample size of 31 was calculated.[20] A total of 45 symptomatic cases of NCC fulfilling the revised Del Brutto’s criteria as shown in Table 1, and 30 controls with non-infectious neurological conditions, without any clinical-radiological evidence of NCC and negative for cysticercus IgG serology, were enrolled in this study, as shown in Figure 1. Radiological scans were used to diagnose NCC based on the revised diagnostic criteria suggested by Del Brutto et al.[21] Adequate clinical details were collected, including age, sex, presenting clinical features, duration and course of symptoms, operative details, and clinical-radiological findings. Blood samples were aseptically obtained from all enrolled participants and stored at −80°C until needed. Inclusion criteria for NCC cases included age ≥18 years, presence of clinical features such as headache or seizures, CT/MRI evidence of NCC lesions, and fulfillment of the revised Del Brutto’s criteria, while exclusion criteria included other CNS infections, prior NCC treatment, and inadequate clinical data.
| Category | Criteria |
|---|---|
| A) Absolute criteria | |
| 1. Histological evidence | Biopsy of a brain or spinal cord lesion showing scolex with suckers and hooks, or parasitic membranes. |
| 2. Imaging confirmation | Cystic lesions showing the scolex as hole-with-dot on CT or MRI. |
| 3. Ophthalmologic finding | Direct visualization of subretinal parasites by funduscopic examination. |
| B) Major criteria | |
| 1. Neuroimaging suggestive of NCC | Cystic lesions without a scolex, single or multiple ring/nodular enhancing lesions, and parenchymal round calcifications. |
| 2. Serology | Positive serum enzyme-linked immunoelectrotransfer blot (EITB) assay for detection of antibodies to T. solium glycoprotein antigens. |
| 3. Spontaneous lesion resolution | Small single enhancing lesions resolving without treatment. |
| 4. Treatment response | Resolution of intracranial cystic lesions after therapy with albendazole or praziquantel. |
| C) Minor criteria | |
| 1. Compatible neuroimaging findings | Hydrocephalus, leptomeningeal enhancement, multiple filling defects, ventricular cysts, ependymitis, and arachnoiditis. |
| 2. Clinical signs | Seizures, focal neurologic deficits, increased intracranial pressure, and intellectual deterioration. |
| 3. CSF findings | Positive CSF ELISA for anticysticercal antibodies or cysticercal antigens. |
| 4. Evidence outside CNS | Presence of cysticercosis in other tissues. |
| D) Epidemiologic criteria | |
| 1. Endemic exposure | Living in or originating from a cysticercosis-endemic region. |
| 2. Travel history | Frequent travel to endemic areas. |
| 3. Household exposure | Close contact with a person with T. solium infection. |
| E) Revised degrees of diagnostic certainty | |
| 1. Definitive Diagnosis | Presence of one absolute criterion OR two major plus one minor and one epidemiologic criterion. |
| 2. Probable Diagnosis | Presence of one major plus two minor criteria OR one major plus one minor and one epidemiologic criterion OR three minor plus one epidemiologic criterion. |
CT: Computed tomography, MRI: Magnetic resonance imaging, NCC: Neurocysticercosis, CSF: Cerebrospinal fluid, ELISA: Enzyme-linked immunosorbent assay, CNS: Central nervous system, T. solium: Taenia solium
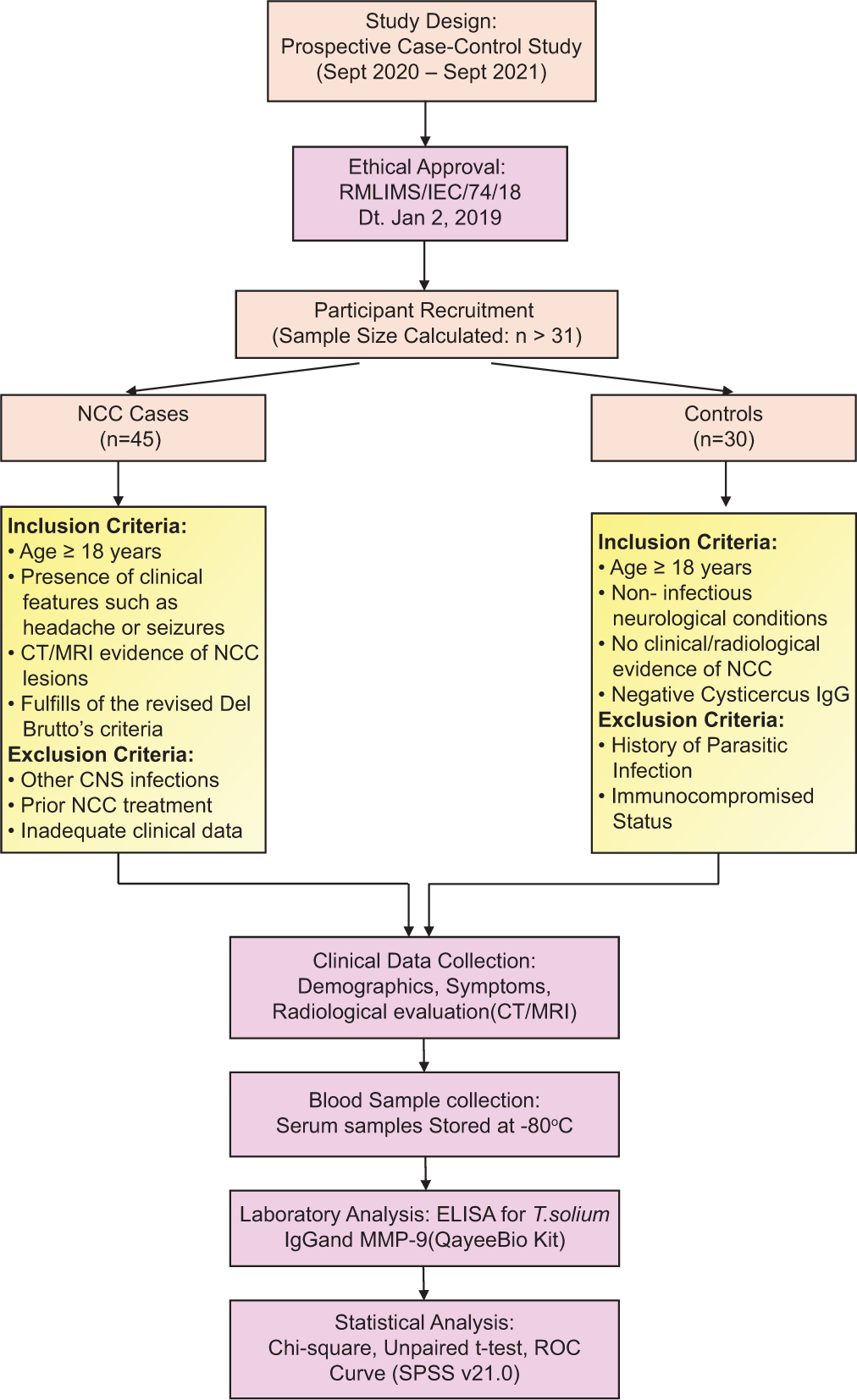
- Flowchart of samples processed in this study. CT: Computed tomography, MRI: Magnetic resonance imaging, NCC: Neurocysticercosis, CSF: Cerebrospinal fluid, ELISA: Enzyme-linked immunosorbent assay, CNS: Central nervous system, MMP-9: Matrix metalloproteinases-9, IgG: Immunoglobulin G, T. solium: Taenia solium, ROC: Receiver operating characteristic, SPSS: Statistical package for the social sciences
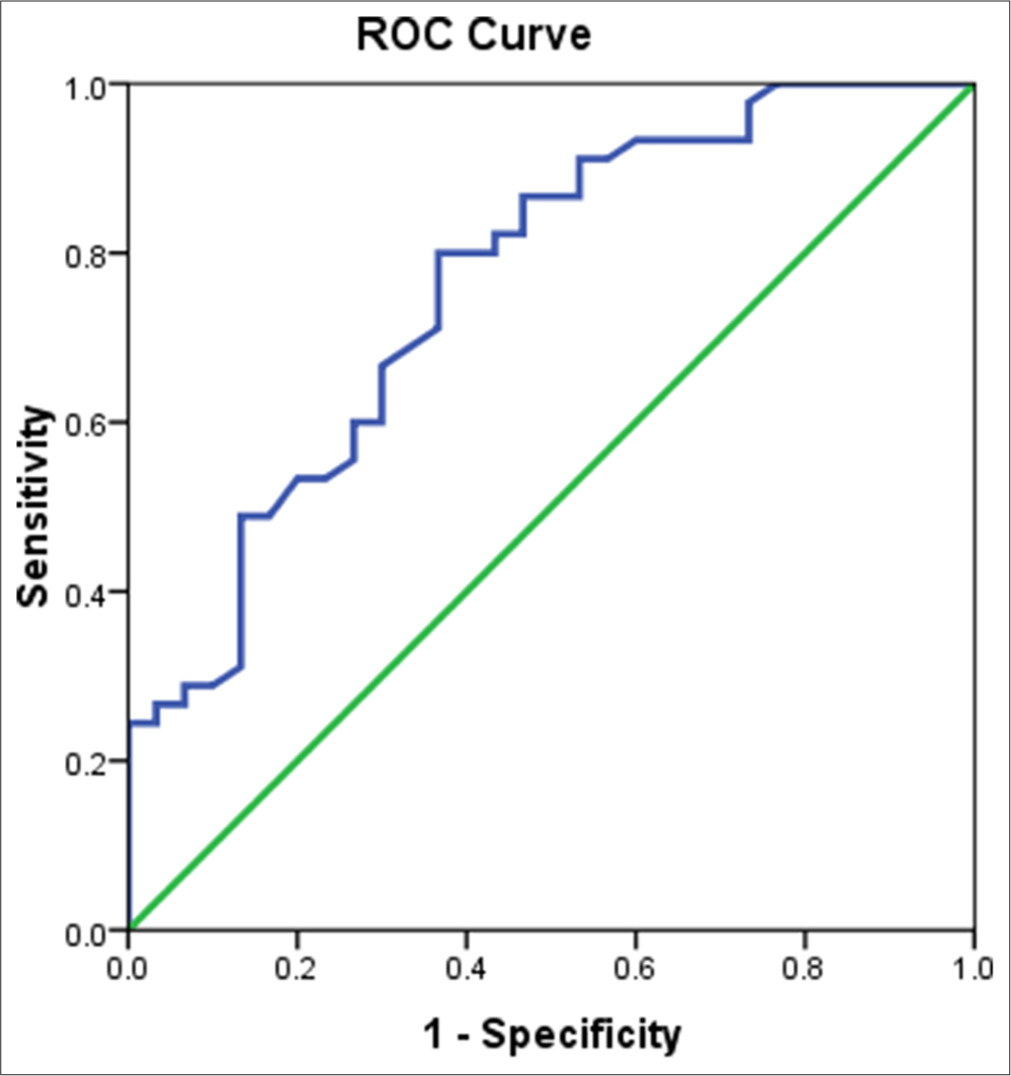
- Receiver operating characteristics curve comparing the matrix metalloproteinase-9 level between cases and controls. ROC: Receiver operating characteristic, Green line: Reference line, Blue line: Matrix metalloproteinase-9
Controls were selected based on the inclusion criteria of age ≥18 years, presence of non-infectious neurological conditions, absence of clinical or radiological evidence of NCC, and negative cysticercus IgG serology. The exclusion criteria for controls included a history of parasitic infections and the presence of other causes of space-occupying lesions, which were ruled out by appropriate clinical and radiological findings. These included conditions such as Toxoplasmosis (radiologically presenting as multiple ring-enhancing lesions with edema, often located in the basal ganglia or corticomedullary junction), tuberculoma (characterized by single or multiple well-defined, ring-enhancing lesions with central necrosis, typically located in the parenchyma or periventricular region), and fungal infections which typically appear as multiple poorly defined, non-enhancing lesions or singular large lesions with central necrosis and irregular borders, often in immunocompromised individuals.
An ELISA kit for T. solium IgG (DRG International Inc., New Jersey, USA) was utilized to detect anti-cysticercus IgG antibodies in all collected sera, adhering to the manufacturer’s instructions. For observing the serum levels of MMP-9, a commercially procured ELISA kit (QayeeBio, Shanghai, China) was employed in all the collected sera following manufacturer instructions. A single-blind test procedure was used for analyzing the serum samples. Those doing the various tests on the specimens were unaware of their origins, including whether they came from patients or control participants.
Statistical analysis
The findings were presented as mean ± standard deviation (SD), frequencies, and percentages. Categorical variables were compared using the Chi-square test, while the unpaired t-test was employed to compare continuous variables. Receiver operating curve (ROC) analysis included calculations of the area under the curve, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and their corresponding 95% confidence intervals (CIs). P < 0.05 was considered significant. The Statistical Package for the Social Sciences version 21.0 (IBM Inc., Chicago, USA) was used for all analyses.
RESULTS
The mean age of cases and controls was 31.20 ± 11.98 and 55.40 ± 9.80 years, respectively. Out of the 45 cases included in this study, 26 (58%) were male and 19 (42%) were female, whereas out of 30 controls, 17 (58%) were male and 13 (42%) were female. Al7l the symptomatic cases have a history of either headache (24%), seizures (62%), or both (14%). In this study, the granular nodular lesions with inflammation and perilesional edema were seen in the majority, that is, 23 (51%) cases, and calcification around the cyst was present in 15 (33.3%) cases. Out of 45 cases of NCC, 30 had probable diagnostic certainty (67%), and 15 had definite diagnostic certainty (33%) as per Del Brutto’s criteria. The demographic and clinical profile of the cases included in this study is shown in Table 2.
| Serial No. | Characteristic | Cases n (%) |
|---|---|---|
| 1 | Gender | |
| Male | 26 (58) | |
| Female | 19 (42) | |
| 2 | Place of residence | |
| Rural | 21 (47) | |
| Urban | 24 (53) | |
| 3. | Clinical features | |
| Headache | 11 (24) | |
| Seizure | 28 (62) | |
| Headache and seizure | 6 (14) | |
| 4. | Dietary habit | |
| Vegetarian | 22 (49) | |
| Non-vegetarian | 23 (51) | |
| 5. | Basic sanitary habit | |
| Open field defecation | 12 (27) | |
| Sanitary latrine | 33 (73) | |
| 6. | History of passing Taenia segment in stool | |
| Yes | 0 | |
| No | 45 (100) | |
| 7. | Prior antibiotic/anthelmintic use | |
| Yes | 0 | |
| No | 45 (100) | |
| 8. | Radiology suggestive of NCC’ | |
| Yes | 26 (58) | |
| No | 19 (42) | |
| 9. | Perilesional edema | |
| Present | 23 (51) | |
| Absent | 22 (49) | |
| 10. | Calcification around cyst | |
| Present | 15 (33) | |
| Absent | 30 (67) | |
| 11. | T. solium IgG ELISA | |
| Positive | 7 (16) | |
| Negative | 38 (84) | |
| 12. | Degree of diagnostic certainty as per Del Brutto's criteria | |
| Probable | 30 (67) | |
| Definite | 15 (33) | |
A ROC curve was generated to compare the mean ± SD values MMP-9 in NCC cases and controls, as shown in Figure 2. The mean ± SD values of MMP-9 were significantly higher (P < 0.00001) in NCC cases (13.42 ± 5.70 ng/mL) compared to healthy controls (8.49 ± 4.21 ng/mL). The area under the curve (95% CI) was 0.76 (0.65–0.87), at a cutoff of >10 ng/mL, serum MMP-9 levels correctly identified that 73.3% of the 45 confirmed NCC cases (33/45; sensitivity) and correctly excluded 63.3% of the 30 controls (19/30; specificity). This threshold resulted in a PPV of 75.0% and an NPV of 61.3%. The diagnostic values of serum human MMP-9 levels in cases from controls are shown in Table 3.
| Serum human MMP-9 level cutoff | Cases | Controls | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| >10.00 ng/mL | 33 | 73.3 | 11 | 36.7 |
| ≤10.00 ng/mL | 12 | 26.7 | 19 | 63.3 |
| Total | 45 | 100 | 30 | 100 |
| Parameter | Diagnostic value | |||
| AUC | 0.76 (0.65-0.87) | |||
| Sensitivity | 73.3 (60.4-86.3) | |||
| Specificity | 63.3 (46.1-80.6) | |||
| PPV | 75.0 (62.2-87.8) | |||
| NPV | 61.3 (44.1-78.4) | |||
MMP-9: Matrix Metalloproteinases-9, AUC: Area under the curve, PPV: Positive predictive value, NPV: Negative predictive value
DISCUSSION
NCC has a diverse clinical presentation and is a slowly progressive disease, with active seizures being the most common manifestation. In this study, seizures were the most common clinical feature, observed in the majority (75%) of cases, which is in agreement with several previous studies reporting a high rate of epilepsy among symptomatic NCC patients, ranging from 70% to 90%.[22-24]
In this study, we observed that 22 (49%) cases were vegetarian, so we can say that both vegetarians and non-vegetarians are vulnerable to acquiring NCC. Studies have shown that consuming improperly cleaned raw or undercooked vegetables or salad is associated with an increase in the chance of getting infection with T. solium eggs.[25] Active case finding, detection of carriers, and treatment of both cases and carriers are necessary for the prevention of NCC.
In this study, radiology suggestive of NCC was present in 26 (57.8%) cases. Neuroimaging with contrast-enhanced computerized tomography or MRI is the cornerstone of NCC diagnosis. CT and MRI scans nearly always show the presence of NCC lesions; however, there may be diagnostic challenges in distinguishing NCC from other brain lesions, such as gliomas, hydatid cysts, tuberculomas, and other parasitic cysts.
In the present study, T. solium IgG ELISA was positive in only 7 (15.6%) cases, which could be due to the poor efficacy and performance of ELISA and other serological test for diagnosis of NCC, as shown by Proaño-Narvaez et al. in their study.[26] The efficacy of serological tests is influenced by the quantity and nature of lesions, with greater sensitivity observed in individuals with multiple lesions compared to those with a solitary cyst. Conversely, individuals with calcified cysts tend to exhibit lower sensitivity, as noted in Garcia et al.’s research.[27] The limited commercial availability and high cost of immunological tests when available are major obstacles to their application in clinical settings.
In this study, we found that serum MMP-9 level was significantly higher (P < 0.00001) among cases (13.42 ± 5.70 ng/mL) compared to controls (8.49 ± 4.21 ng/mL), which was in accordance with an Indian study done by Verma et al. on 36 symptomatic patients with active seizures and 37 controls where they reported a significantly higher (P < 0.001) concentration of MMP-9 in symptomatic cases compared to control.[28] In this study, serum MMP-9 level of >10 ng/mL correctly predicted NCC in 73.3% of NCC cases with a specificity of 63.3%. Studies done by Alvarez and Teale have shown that MMPs play a crucial role in the pathophysiology of numerous CNS infections, including the NCC murine model.[29,30] MMP-9 is known to contribute significantly to the disruption of the BBB, facilitating the extravasation of monocytes and T lymphocytes into the CNS.[31] Increased MMP-9 levels in symptomatic NCC patients may explain the high frequency of seizures in the cases enrolled in this study. Similarly, Wilczynski et al. found that MMP-9 is responsible for repeated epileptic attacks in animal models.[32]
When comparing the sensitivity of MMP-9 ELISA with other diagnostic methods for NCC, several recent studies highlight the variability and strengths of alternative tests. The EITB demonstrates high sensitivity (81.1–100%) for detecting parenchymal active multiple cysts, although sensitivity drops significantly for single cysts (<62.6%).[33] Polymerase chain reaction (PCR) is a highly specific method that can detect T. solium DNA in CSF, serum, or tissue samples. According to Carpio et al., PCR had a sensitivity of 72.2% and a specificity of 100.0%.[34] Similarly, Yera et al. reported a sensitivity and specificity of 83.3% and 100% for PCR assay detecting NCC.[35] In comparison to other diagnostic methods, MMP-9 ELISA’s sensitivity of 73.3% places it in a similar sensitivity range. Thus, MMP-9 ELISA could serve as an adjunctive diagnostic tool, particularly for cases where conventional methods are inconclusive or difficult to access, offering additional insights into the pathophysiological mechanisms of NCC. Combining it with other diagnostic techniques could improve overall diagnostic accuracy, particularly in areas where neuroimaging or serology are less feasible.
Studies have demonstrated that higher serum MMP-9 levels correlate with symptomatic NCC, suggesting a link between MMP-9 activity and disease severity.[15,28] Furthermore, dynamic contrast-enhanced MRI evaluations have revealed that MMP-9 levels vary across different stages of NCC, being most pronounced in the colloidal stage and least in the calcified stage.[36] This variability underscores the potential of MMP-9 as a biomarker for disease progression. Monitoring MMP-9 levels provides valuable insights into treatment efficacy and the risk of long-term neurological sequelae, facilitating more personalized therapeutic strategies.
MMP-9 levels could be measured early alongside standard serological tests to identify active NCC cases, particularly in patients with inconclusive imaging or negative serology. In cases where NCC is suspected but radiological or serological results are ambiguous, elevated MMP-9 could help confirm the diagnosis and differentiate it from other conditions such as gliomas, tuberculomas, or hydatid cysts. In addition, as MMP-9 correlates with disease severity and inflammation, it could be used to monitor disease progression or response to treatment, especially in cases with multiple cysts or active inflammation, where tracking BBB disruption is crucial.
Limitations of the study
This study has several limitations, including its single-center, hospital-based design with a limited sample size. Serum MMP-9 levels were assessed only at baseline, without evaluation at different time points or correlation with symptom resolution. Age differences between cases and controls were not adjusted due to the small sample size, which may influence MMP-9 levels. In addition, the control group included non-infectious neurological cases, some of which could inherently alter MMP-9 levels.
CONCLUSIONS
In this study, we observed that serum MMP-9 was capable of discriminating NCC cases from controls with a sensitivity and specificity of 73 and 63%, respectively. While MMP-9 may have potential diagnostic value, its moderate sensitivity and specificity suggest it should not be used as a standalone marker. Instead, it could complement existing serological and imaging tools to improve NCC diagnosis. Further research is necessary to validate these results, ascertain the significance of serum MMP-9 as both a prognostic and diagnostic biomarker in NCC, and explore alternative markers in larger, multicenter studies.
Author contribution
NR and MY: Contributed to the study design, literature search, manuscript preparation, editing, and review; MS and AD: Provided intellectual content definition and performed data analysis; AKS: Was responsible for data acquisition; JA: Contributed to the study design and manuscript editing.
Ethical approval
The research/study was approved by the Institutional Review Board at Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, number RMLIMS/IEC/74/18, 2nd January 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship: Nil.
References
- Taenia solium cysticercosis and its impact in neurological disease. Clin Microbiol Rev. 2020;33:e00085-19.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocysticercosis in patients with active epilepsy in the tea garden community of Assam, Northeast India. Sci Rep. 2021;11:7433.
- [CrossRef] [PubMed] [Google Scholar]
- Taeniasis/cysticercosis. Available from: https://www.who.int/news-room/factsheets/detail/taeniasis-cysticercosis [Last accesed 2024 Sep 01]
- [Google Scholar]
- Unique characteristics of epilepsy development in neurocysticercosis. Am J Trop Med Hyg. 2020;103:639-45.
- [CrossRef] [PubMed] [Google Scholar]
- The causal relationship between neurocysticercosis infection and the development of epilepsy-a systematic review. Infect Dis Poverty. 2017;6:31.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocysticercosis: Diagnostic problems and current therapeutic strategies. Indian J Med Res. 2016;144:319-26.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164:1007-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202-15.
- [CrossRef] [PubMed] [Google Scholar]
- Enzyme-linked immunosorbent assay (ELISA) for the detection of antibody to cysticerci of Taenia solium. Am J Trop Med Hyg. 1982;31:364-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Significance of matrix metalloproteinases in parasitic infections involving the central nervous system. Pathogens. 2013;2:105.
- [CrossRef] [PubMed] [Google Scholar]
- The role of matrix metalloproteinase in inflammation with a focus on infectious diseases. Int J Mol Sci. 2022;23:10546.
- [CrossRef] [PubMed] [Google Scholar]
- Recent advances in understanding the human host immune response in tuberculous meningitis. Front Immunol. 2024;14:1326651.
- [CrossRef] [PubMed] [Google Scholar]
- Blood-brain barrier integrity damage in bacterial meningitis: The underlying link, mechanisms, and therapeutic targets. Int J Mol Sci. 2023;24:2852.
- [CrossRef] [PubMed] [Google Scholar]
- MMPs/TIMPs imbalances in the peripheral blood and cerebrospinal fluid are associated with the pathogenesis of HIV-1-associated neurocognitive disorders. Brain Behav Immun. 2017;65:161-72.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokines, MMP-2, and MMP-9 levels in patients with a solitary cysticercus granuloma. Neurol India. 2015;63:190-6.
- [CrossRef] [PubMed] [Google Scholar]
- Potential genetic polymorphism of matrix metalloproteinase (MMP)-9 in Iranian migraine patients with Toxoplasma gondii infection. Parasitol Res. 2024;123:140.
- [CrossRef] [PubMed] [Google Scholar]
- The Activity of matrix metalloproteinases (MMP-2, MMP-9) and their tissue inhibitors (TIMP-1, TIMP-3) in the cerebral cortex and hippocampus in experimental acanthamoebiasis. Int J Mol Sci. 2018;19:4128.
- [CrossRef] [PubMed] [Google Scholar]
- Neurological disease in human and canine leishmaniasis--clinical features and immunopathogenesis. Parasite Immunol. 2015;37:385-93.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of MMP-9 levels in the serum of patients with epilepsy. Front Neurosci. 2024;18:1296876.
- [CrossRef] [PubMed] [Google Scholar]
- Active epilepsy as an index of the burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135-9.
- [CrossRef] [PubMed] [Google Scholar]
- Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. 2017;372:202-10.
- [CrossRef] [PubMed] [Google Scholar]
- Active neurocysticercosis, parenchymal and extraparenchymal: A study of 38 patients. J Neurol. 1993;241:15-21.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocysticercosis: Clinical, radiologic, and inflammatory differences between children and adults. Pediatr Infect Dis J. 2006;25:801-3.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocysticercosis--a review of 231 cases. Infection. 1992;20:61-5.
- [CrossRef] [PubMed] [Google Scholar]
- The survival and dispersal of Taenia eggs in the environment: What are the implications for transmission? A systematic review. Parasit Vectors. 2021;14:88.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory diagnosis of human neurocysticercosis: Double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol. 2002;40:2115-8.
- [CrossRef] [PubMed] [Google Scholar]
- Neurocysticercosis: Is serology useful in the absence of brain imaging? Trop Med Int Health. 2012;17:1014-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology. 2011;138:1423-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence for differential changes of junctional complex proteins in murine neurocysticercosis dependent upon CNS vasculature. Brain Res. 2007;1169:98-111.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple expression of matrix metalloproteinases in murine neurocysticercosis: Implications for leukocyte migration through multiple central nervous system barriers. Brain Res. 2008;1214:145-58.
- [CrossRef] [PubMed] [Google Scholar]
- Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006;44:1105-14.
- [CrossRef] [PubMed] [Google Scholar]
- Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol. 2008;180:1021-35.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy of immunological tests on serum and urine for diagnosis of Taenia solium neurocysticercosis: A systematic review. PLoS Negl Trop Dis. 2024;18:e0012643.
- [CrossRef] [PubMed] [Google Scholar]
- Validity of a PCR assay in CSF for the diagnosis of neurocysticercosis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e324.
- [CrossRef] [PubMed] [Google Scholar]
- Confirmation and follow-up of neurocysticercosis by real-time PCR in cerebrospinal fluid samples of patients living in France. J Clin Microbiol. 2011;49:4338-40.
- [CrossRef] [PubMed] [Google Scholar]
- T1-weighted dynamic contrast-enhanced MR evaluation of different stages of neurocysticercosis and its relationship with serum MMP-9 expression. AJNR Am J Neuroradiol. 2013;34:997-1003.
- [CrossRef] [PubMed] [Google Scholar]






