Translate this page into:
A clinical decision rule for streptococcal pharyngitis management: An update
Address for correspondence: Dr. Saeedeh Tarvijeslami, Zargande, Javahery Hospital, Department of Pediatrics, Tehran, Iran. E-mail: s_tarvij@yahoo.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
PURPOSE:
Group A streptococcal (GAS) pharyngitis is a common disease worldwide. We aimed to establish a pragmatic program as a clinical decision rule for GAS pharyngitis diagnosis.
MATERIALS AND METHODS:
This article derived from a research project on children aged 6–15 years. Five hundred and seventy-one children met the enrollment criteria on whom throat culture and validities of clinical findings were assessed in positive and negative throat culture groups.
RESULTS:
Positive GAS throat culture group included 99 (17.3%) patients with a positive culture. Negative GAS throat culture group included 472 (82.6%) patients. Exudate or enlarged tender nodes each one had 63% and 68% sensitivity and 31.5% and 37.5% specificity with a high percentage of negative predictive value (NPV) 80.54% and 85.09%, respectively. Sequence test revealed validities of exudate plus enlarged nodes at 43.62% sensitivity and 57.19% specificity with 83% NPV.
CONCLUSIONS:
High NPV of 83% indicated that similar prevalence in the absence of either exudate or enlarged tender lymph nodes. Probability of GAS negative throat cultures among children suspected of GAS pharyngitis was 83% and would correctly not receive inopportune antibiotics.
Keywords
Erythema
exudate
lymph nodes
pharyngitis
streptococcal
Introduction
Group A beta-hemolytic streptococcal (GAS) pharyngitis (strep throat) is a common disease worldwide with higher incidence at 3–15-year-old children, especially in young school-aged children.[1] About one in four children with an acute sore throat has serologically confirmed GAS pharyngitis. Forty-three percent of families with an index case of GAS pharyngitis have a secondary case.[2] GAS is responsible for 5–15% of sore throat visits in adults and 20–30% in children.[12] GAS pharyngeal infection not only causes acute illness but also can trigger the postinfectious syndromes of poststreptococcal glomerulonephritis and acute rheumatic fever (ARF) that is currently uncommon in most developed countries, but it remains the leading cause of acquired heart disease, rheumatic mitral stenosis that is a common sequel in the first and early second decades of life among children in many resource-poor areas.[3] ARF incidence rates 50–100 times higher in low-income countries versus high-income regions, therefore diagnosis and treatment of GAS pharyngitis is very important. Most high-income regions practice the primary prevention of ARF, laboratory diagnosis of streptococcal pharyngitis, followed by antibiotics. This strategy is not practical for low-income regions because of the high cost or unavailability of diagnostic testing. As diagnosis depends on clinical signs, which their positive predictive values (PPVs) are low, the clinicians may presumptively empirically treat all the patients with antibiotics.[456789] In most clinical studies, GAS was cultured in 20–30% of all acute pharyngitis cases in children indicating that treatment of all pharyngitis is excessive using a variety of clinical rules to make decision for treatment without laboratory data may be considered and a CDR should be established in these regions.[101112]
We aimed to establish and suggest an easy, safe, pragmatic, clinical combination of scoring system, an updated CDR for the prediction of diagnosis of GAS pharyngitis that could help clinicians to exclude GAS infection in children with pharyngitis and would help avoid unnecessary treatment with antimicrobial agents in low-resource settings, where there is poor access to laboratory support, culture, and rapid antigen detection test (RDT), and rheumatic fever may remain a major public issue.
Materials and Methods
We should explain that this article and the similar article[13] were derived from a research project that had been funded by a grant from the research and education foundation of our university with the code: NCT01310361.
As the source of the both articles is the same, the sample size and a part of methodology and results are similar, but with a different background, objective, final results, and different aspects and aims.
This research project as a cross-sectional study performed on 6–15-year-old elementary and high-school children from seven different regions of North-East of Iran, Khorasan, Mashhad, from March 2012 to March 2013.[13] They were screened for enrollment as pharyngitis with clinical criteria of sore throat, erythema, exudate, and tender or enlarged anterior cervical lymph nodes (larger than 1.5 cm). Exclusion criteria were considered: Oral antibiotics use within the preceding 3 days or intramuscularly administered antibiotics within the 28 days prior to the visit which may modify clinical presentation of streptococcal pharyngitis, history of previous ARF or rheumatic heart disease, presence of another illness, and to be carrier.[1314] The method of sampling was nonprobability, easy sampling. When we considered P < 0.05, confidence level 95%, and permissible error 1%, at least 97 children with GAS positive throat culture were expected as sample size. Five hundred and eighty-five children met the enrollment criteria but about 14 patients refused the study and were excluded and 571 patients participated in the study. The children were compared with respect to age, gender, and other variables. The patients at schools were introduced by a health-care worker. The medical history and general health status of each participant were observed and assessed by a physician. For making a diagnosis, we used the gold standard method; bacterial throat culture on blood agar medium with 90–95% sensitivity. We did not RDT strategy that its negative results should be confirmed by culture and has a median false-negative rate of 11%.[131516] The physician applied throat culture soaps that were sent to a reference laboratory. Ninety-nine patients had throat swabs that yielded positive results on culture.[713] Patients were observed before obtaining the culture and in GAS positive, negative throat culture groups again, and clinical signs, and symptoms were recorded and compared.
The final outcome assessed on the basis of sensitivity and specificity as the main values to establish CDR, and also PPV and negative predictive value (NPV) were considered compared with throat culture as well. Although PPV and NPV vary with prevalence, they are of interest to clinicians. As PPV is usually low for clinical signs,[14] we considered NPV as well. A meta-analysis confirmed that symptoms alone were not sufficient to rule out this diagnosis. Because the individual variables predict GAS pharyngitis so poorly, researchers have suggested combining potential predictive variables within a CDR.[16] In the present study, the validity of each sign and symptom separately and in combinations (two-variable rules) according to the prevalence of positive throat culture was calculated by parallel test and sequences test, and the percentage of sensitivity, specificity, PPV, and NPV were evaluated.
For the comparison of results, t-test was used and for the quality variants, Fisher and Chi-square tests were used with P < 0.05 through the SPSS 17.0 (SPSS Inc, Illinois, USA).
Ethical considerations
This study was conducted according to the Ethical Standards Guidelines of Helsinki 1964 the Guidelines for the Ethical Conduct of Medical Research Involving Children revised by the Royal College of Pediatrics and Child Health: Ethics Advisory Committee. We considered Committee on Publication Ethics guidelines as well. All the cooperators and the parents were explained about the mentioned method. We received written informed consent from all patients and from education organization as well.
Results
Of the 571 children (225 male and 346 female) who participated in the study, 99 and 472 patients’ throat swabs yielded positive and negative GAS throat culture, respectively.
The percentage of positive throat culture was 17.3%, of which 51 (51.5%) were male and 48 (48.5%) were female. Male to female ratio was 1.8 in positive culture group (P = 0.07).[13] There was no significant difference between clinical signs and symptoms in negative and positive throat culture group patients (P > 0.05).
Signs and symptoms of patients with positive and negative throat culture before treatment and the validities of each sign and symptom alone are shown in Table 1.
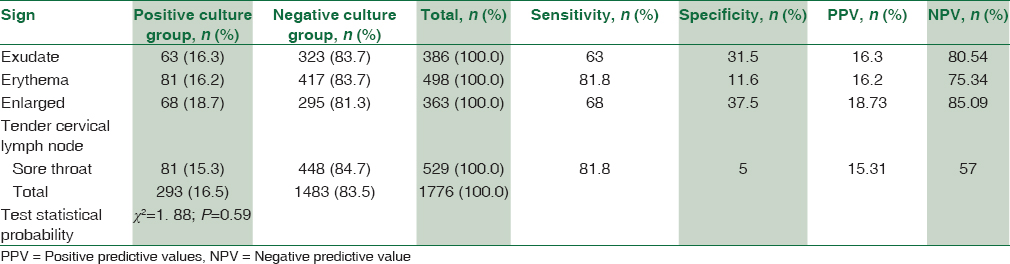
Erythema and sore throat each one was the most common sign in both groups.
Erythema or sore throat each one had high sensitivity 81.8% with low specificity.
Exudate or enlarged tender cervical lymph node each one was 63% and 68% sensitive with moderate specificity, respectively, and high NPV of 80.54% and 85.09%, respectively [Table 1].
The validities of sign and symptom in combinations (two-variable rules) according to the prevalence of positive throat culture (17.3%) were calculated as follows [Table 2].
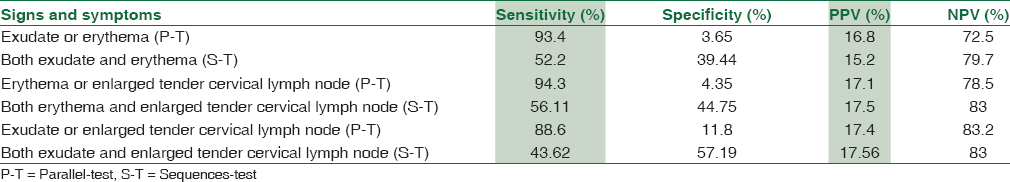
In combination tests of two-variable rules, parallel test enhanced sensitivity but decreased specificity. In sequences test, erythema plus enlarged, tender cervical lymph nodes had 56.11% sensitivity, 44.75% specificity, and high percentage of NPV 83%. Exudate plus enlarged tender cervical lymph nodes had 43.62% sensitivity, 57.19% specificity, and high percentage of NPV, 83% [Table 2].
Discussion
Current management for children and adolescents includes 1 of 6 strategies such as (1) observed without testing or treatment, (2) treat all suspected cases with antibiotic, (3) treat those with positive throat cultures, (4) treat those with positive rapid tests, (6) treat those with positive throat cultures after negative rapid tests, and (6) usage of a clinical scoring measure to determine the diagnosis/treatment strategy.[17]
In most researches, GAS was cultured in 20–30% of all acute pharyngitis in children and it is over diagnosis to treat all pharyngitis is.[1] Although a negative result in many cases of bacterial infection does not exclude an infective etiology, this leaves the physician in a dilemma as to whether the patient needs antibiotics or not[18] but as mentioned before, the laboratory diagnosis strategy of GAS pharyngitis is not practical for low-income countries, and usage of a variety of clinical rules to determine the diagnosis/treatment strategy is more acceptable for low-income countries. The present study is comparable with similar studies, which are regional trials to determine CDR. A combination of symptoms could help clinicians to diagnosis or exclude GAS infection in children with pharyngitis.
Attia et al. reported that posttest probability values for a positive throat culture associated with positive and negative predictors of the model were 79% and 12%, respectively.[19]
In the WHO cohort study among 5-year-old children or older, sensitivity for sore throat was low and ranged from 3.8% in Egypt to 10.8% in Brazil. In children under 5 years, sensitivity was low (0.0–4.6%), and specificity was high in both age groups (93.8 and 97.4%), respectively. A CDR with higher sensitivity should be developed for use in regions where rheumatic fever and rheumatic heart disease are still major health problems.[14]
Sahin et al. study revealed that sensitivities of combinations including two of the following three symptoms, namely sore throat, pharyngeal erythema, and exudate or sore throat and fever were 76.9% and 87.7%, respectively. Specificities of the same combinations were 49.4% and 30.6%, respectively.[20]
Steinhoff et al. reported that exudate or large cervical nodes would be 84% sensitive and 40% specific.[21]
In a randomized, clinical trial for GAS pharyngitis, the integrated clinical prediction rule process for integrating complex evidence-based clinical decision report tools is of relevant importance for nationals.[22]
In a meta-analysis, the validation studies were assessed based on sensitivity, specificity, and positive likelihood ratio for non-GAS infections with the clinical approach, compared with a throat culture or RDT results.
In the meta-analysis of the validation studies, symptoms alone were not sufficient to rule out this diagnosis. To identify the best CDR for diagnosing GAS pharyngitis in children, a combination of symptoms could help clinicians exclude GAS infection.[16]
In our study, 571 children were included among whom 99 and 472 patients’ throat swabs yielded positive and negative GAS throat culture, respectively. Exudate or enlarged tender cervical lymph node each one with 63% and 68% sensitivity, 31.5% and 37.5% specificity, and the high percentage of NPV, 80.54% and 85.09%, respectively, indicated that in the present of exudate or large cervical nodes, about 68 of 99 patients with positive throat culture would correctly receive antibiotics and about 174 of 472 patients with negative throat culture would correctly not receive antibiotics (reduction of unnecessary antibiotic treatment by our CDR).
High NPV percentage of 80.54% and 85.09% means that in a population as our study which prevalence of GAS pharyngitis is 17.3% (compatible with many studies), in a child suspected of GAS pharyngitis and in the absence of exudate, probability of GAS negative throat culture would be 80.54% and in the absence of large cervical nodes, probability of GAS negative throat culture would be 85.09% and would not correctly receive inopportune antibiotics, and our CDR could reduce unnecessary antibiotic treatment in a low-resource setting.
In combination tests of two indices, parallel-test enhances sensitivity but decreases specificity.
In sequences test, we offer two following guidelines which suggest treatment:
Exudate plus enlarged tender cervical nodes were 43.62 sensitive and 57.19 specific, based on them, about 44/99 children with positive throat culture would correctly receive antibiotics and 274/472 children with negative throat culture would correctly not receive antibiotics (reduction in unnecessary antibiotic treatment). Exudate plus enlarged nodes had the highest percentage of NPV, 83%.
Erythema plus enlarged tender cervical nodes were 56.11% sensitive and 44.75% specific that indicated about 56/99 children with positive throat culture would correctly receive antibiotics and about 212/472 children with negative throat culture would correctly not receive antibiotics (reduction in unnecessary antibiotic treatment), with the highest percentage of NPV, 83%, which indicated that in a prevalence of GAS pharyngitis similar to our study (17.3%), in the absence of either exudate or enlarged tender lymph nodes or in the absence of either erythema or enlarged tender lymph nodes, probability of GAS negative throat culture was 83% among children suspected of GAS pharyngitis and would correctly not receive inopportune antibiotics (reduction in unnecessary antibiotic treatment).
The present CDR could be helpful in reducing antibiotic abstention for pharyngitis in children in low-resources settings. As well as it might be useful for countries where the RDT use is not recommended in current practice.[18] It might also well interest the 50% of physicians who do not use RDTs at all.[16]
Although in different studies, similar criteria were included to establish CDR, the rate of validities of sign and symptom and percentage of positive GAS throat culture were different. These differences indicated variation in clinical manifestations of pharyngitis by region. However, additional studies from other regions are necessary before modified guidelines are implemented.
Conclusions
High NPV of 83% indicated that in similar prevalence in the absence of either exudate or enlarged tender lymph nodes. Probability GAS negative throat culture among children suspected of GAS pharyngitis was 83% and would correctly not receive inopportune antibiotics.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical practice guideline for the diagnosis and management of Group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:e86-102.
- [Google Scholar]
- Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;1(79):383-90.
- [Google Scholar]
- Treatment of children with rheumatic heart disease in Sub-Saharan Africa by overseas’ medical missions: Challenges left behind. J Cardiol Clin Res. 2014;2:1016.
- [Google Scholar]
- Prevalence of streptococcal pharyngitis and streptococcal carriage in children: A meta-analysis. Pediatrics. 2010;126:e557-64.
- [Google Scholar]
- Group A streptococcal infection. In: Report of the Committee on Infectious Diseases. Red Book (28th ed). Elk Grove Village, IL: American Academy of Pediatrics; 2009. p. :616-7.
- [Google Scholar]
- Group A Streptococcus. In: Kliegman RM, Stanton BF, Geme ST, Schor NF, Behrman RE, eds. Nelson Text Book of Pediatrics (19th ed). Philadelphia: Elsevier; 2011. p. :914-24.
- [Google Scholar]
- Re-evaluation of antibiotic treatment of streptococcal pharyngitis. Curr Opin Pediatr. 2010;22:77-82.
- [Google Scholar]
- Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of Group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis. 2002;35:113-25.
- [Google Scholar]
- A clinical decision rule for management of streptococcal pharyngitis in low-resource settings. Acta Paediatr. 2005;94:1038-42.
- [Google Scholar]
- Pharyngitis in low-resources settings: A pragmatic clinical approach to reduce unnecessary antibiotic use. Pediatrics. 2006;118:e1607-11.
- [Google Scholar]
- Comparing performance of amoxicillin and intramuscular benzathine penicillin in relieving manifestations of streptococcal pharyngitis in children. Ghana Med J. 2014;48:185-8.
- [Google Scholar]
- Evaluation of the WHO clinical decision rule for streptococcal pharyngitis. Arch Dis Child. 2005;90:1066-70.
- [Google Scholar]
- Throat culture is necessary after negative rapid antigen detection tests. Clin Pediatr (Phila). 2007;46:241-6.
- [Google Scholar]
- Streptococcal pharyngitis in children: A meta-analysis of clinical decision rules and their clinical variables. BMJ Open. 2013;3 pii: e001482
- [Google Scholar]
- Diagnosis and management of pharyngitis in a pediatric population based on cost-effectiveness and projected health outcomes. Pediatrics. 2006;117:609-19.
- [Google Scholar]
- Associations between procalcitonin and markers of bacterial sepsis. Medicina (Kaunas). 2012;48:383-7.
- [Google Scholar]
- Performance of a predictive model for streptococcal pharyngitis in children. Arch Pediatr Adolesc Med. 2001;155:687-91.
- [Google Scholar]
- The validity of diagnostic criteria for streptococcal pharyngitis in integrated management of childhood illness (IMCI) guidelines. J Trop Pediatr. 2003;49:377-9.
- [Google Scholar]
- Effectiveness of clinical guidelines for the presumptive treatment of streptococcal pharyngitis in Egyptian children. Lancet. 1997;350:918-21.
- [Google Scholar]
- Efficacy of an evidence-based clinical decision support in primary care practices: A randomized clinical trial. JAMA Intern Med. 2013;173:1584-91.
- [Google Scholar]





