Translate this page into:
A cytohistopathological correlation study of skin lesions in leprosy: An experience in a tertiary care hospital in South India
*Corresponding author: Dr. Ramya Katta, MD, 203, Sai Nilaya, Nelson Mandela Park Road, LIC Colony, Vijayawada, Andhra Pradesh, India 520008 drkattaramya@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Padma M, Katta R. A cytohistopathological correlation study of skin lesions in leprosy: An experience in a tertiary care hospital in South India. J Lab Physicians. 2024;16:170-5. doi: 10.1055/s-0043-1771020
Abstract
Objectives:
Leprosy is a granulomatous disease caused by Mycobacterium leprae. Early detection and clinical diagnosis remain a challenge, especially in paucibacillary cases and initial lesions during early phases of leprosy. Use of cytological methods can reduce time for diagnosis, especially in peripheral centers. The present study was taken up to calculate the correlation between cytology and histopathology of skin lesions in leprosy.
Materials and Methods:
This study is a 2-year retrospective observational study taken up in the Department of Pathology, Rangaraya Medical College and Government General Hospital, Kakinada, Andhra Pradesh. A total of 49 cases of clinically suspected leprosy were included in the study. Skin biopsies and cytological smears were examined in all cases.
Results:
A total of 49 cases of clinically suspected leprosy were included in the study. The age incidence ranged between 9 and 72 years. There was a male preponderance. The overall diagnostic accuracy of fine-needle aspiration smears was 83.3% and that of slit-skin smears was 62.1%.
Conclusions:
Cytological examination being a simple, less time-consuming, and easy technique can enhance the diagnostic accuracy in detection of leprosy and act as an adjunct to clinical diagnosis.
Keywords
Hansen’s disease
Fite stain
Zeihl–neelsen stain
Ridley–jopling scale
INTRODUCTION
Leprosy or Hansen’s disease is a communicable disease caused by Mycobacterium leprae, a bacteria of low invasive power and pathogenicity.[1] It evokes a granulomatous reaction that can be localized or widespread, self-limiting or progressive, based on the host’s immune response.[2] Although this disease has been known since times unknown, it is still endemic in various states of India, with an annual case detection rate of 4.56 per 10,000 and a prevalence of 0.4 per 10,000 population.[3] East Godavari district in Andhra Pradesh has been categorized as a district of moderate endemicity in the recent surveillance.[3] The disease manifests itself in two polar forms and a borderline type lying in between these forms. Early detection of the disease remains a challenge due to its atypical presentation and overall reduction on the disease incidence.[4,5]
Even though histopathological examination is not mandatory for disease identification, it plays an important role in confirmation and correct typing and hence prognostication of the disease.[5,6] It also helps identify progression and regression of the disease in patients taking therapy.[7]
In leprosy, the utility of fine-needle aspiration cytology and slit-skin smears has been limited mostly to identifying bacteriological and morphological indices.[8,9] However, there are a few studies that indicate the efficacy of these techniques in classifying these lesions in accordance to a modified three-tier classification (tuberculoid, midborderline, and lepromatous) using morphological features of the inflammatory cells.[10,11] The aim of the present study is to identify the concordance between cytological and histopathological diagnosis of leprosy.
MATERIALS AND METHODS
The present study is a retrospective observational study taken up in the Department of Pathology, Rangaraya Medical College and Government General Hospital, Kakinada, Andhra Pradesh during the period from January 2003 to December 2004. New patients who presented to dermatology outpatient unit and satisfying the World Health Organization (WHO) criteria[12] for diagnosis of leprosy were included in the study. The criteria include definite loss of sensation in a pale or reddish skin patch, thickened or enlarged peripheral nerves with loss of sensation, and/or weakness of the muscles supplied by that nerve, and/or microscopic detection of bacilli by slit-skin smears. Patients with previous history of leprosy or who had partial or complete treatment for leprosy were excluded from the study. Institutional ethical clearance was taken (IEC Registration No: ECR/467/lns/AP/2013/RR-19, dated 22-12-20) and consents of the patients or guardians were taken wherever necessary.
After the application of inclusion and exclusion criteria, a total of 49 cases of clinically suspected leprosy were included in the study. All these cases were sent to the Department of Pathology for obtaining skin biopsies and cytological material.
For histopathological examination, in all the cases, irrespective of the type of lesion, 4-mm punch biopsy was taken using skin punch biopsy needle under aseptic precautions. All the skin biopsies obtained were routinely fixed in 10% neutral buffered formalin for a minimum of 6 to 24 hours; processed manually using graded alcohols, xylene, and paraffin wax; and sectioned at 4-µm thickness using Leica manual rotary microtome.[13] Sections were stained with hematoxylin and eosin for morphology and modified Fite stain10 for assessing bacterial index. The Ridley–Jopling (RJ) classification[11,14,15] was used for bacteriological and histological typing in the present study.
For cytological examination, patients with flat lesions were subjected to slit-skin smearing and cases with nodular lesion were subjected to fine-needle aspiration cytology. For slit-skin smears preparation, the slit and scrape method was used[16] and at least two smears were made. Cytological smears were stained using the May–Grunwald–Giemsa stain for morphology and Ziehl–Neelsen (ZN) stain for bacterial index. Singh et al’s[10] cytological criteria with additional modifications by Prasad et al[17] for leprosy classification were used to make cytological diagnosis (Table 1). Smears were considered adequate when either inflammatory infiltrate or skin appendage cells were seen (eccrine glands).
| Types | Cytological features | Acid-fast bacteria (AFB) stain |
|---|---|---|
| Tuberculoid leprosy (TT and BT) | Cellular smears Cohesive epithelioid cell granulomas. Numerous lymphocytes not infiltrating granuloma | No stainable AFB (BI = 0) |
| Borderline leprosy (BB) | Fair cellular yield Poorly cohesive granulomas composed of an admixture of epithelioid cells and macrophages. Few lymphocytes infiltrating the granulomas |
BI = 1þ to 2þ |
| Borderline lepromatous leprosy (BL) | Moderate cellularity Singly dispersed macrophages with “negative images”; no epithelioid cells Numerous lymphocytes (predominant cell type) diffusely admixed with macrophages |
BI = 3þ to 4þ |
| Lepromatous leprosy (LL) | Heavy cellularity Numerous foamy macrophages (predominant cell type) in a fatty background with intracellular and extracellular negative images Few lymphocytes |
BI = 5þ to 6þ (Globi) |
| Histoid leprosy | Cellular yields, elongated spindle cells, scattered lymphocytes | BI = 6þ |
RESULTS
A total of 49 cases of clinically suspected leprosy were included in the study. The age incidence ranged between 9 and 72 years with the most common age group being the third decade of life. There was a male preponderance with a male-to-female ratio of 1.7:1. The most common skin lesions were hypopigmented patches constituting 30 cases. All the cases were further typed along the RJ scale. The most common histopathological diagnosis was borderline tuber-culoid type followed by indeterminate leprosy (Table 2).
| No. of cases | Percentage | |
|---|---|---|
| Age | ||
| 0–10 | 2 | 4.0 |
| 11–20 | 15 | 30.6 |
| 21–30 | 16 | 32.6 |
| 31–40 | 12 | 24.6 |
| 41–50 | 3 | 6.2 |
| 51–60 | 0 | 0 |
| 61–70 | 0 | 0 |
| 71–80 | 1 | 2.0 |
| Gender | ||
| Males | 31 | 63.2 |
| Females | 18 | 36.8 |
| Type of skin lesions | ||
| Hypopigmented patches | 30 | 61.3 |
| Hypopigmented and copper-colored patches | 7 | 14.2 |
| Raised erythematous or nodular lesions | 12 | 24.5 |
| Histopathological diagnosis | ||
| Tuberculoid (TT) | 10 | 20.5 |
| Borderline tuberculoid (BT) | 17 | 34.7 |
| Midborderline (BB) | 0 | 0 |
| Borderline lepromatous (BL) | 3 | 6.1 |
| Lepromatous (LL) | 7 | 14.3 |
| Indeterminate leprosy | 12 | 24.4 |
Slit-skin smears were performed in 37 cases and fine-needle aspiration was performed in 12 cases based on the type of lesion (Tables 3 and 4). Of these, 40 cases showed adequate material on microscopy. It was found that sample inadequacy was more frequent in the cases where slit-skin smears were taken (6 cases). Inadequate material was obtained mainly in cases reported as indeterminate leprosy on histology (6 cases), two cases of borderline tuberculoid, and one case of lepromatous leprosy.
| Histopathological diagnosis | Fine-needle aspiration cytological diagnosis (12 cases) | |||||
|---|---|---|---|---|---|---|
| Type | No. of cases | Tuberculoid (BT and TT) | BB/BL | BL | LL | Inadequate material |
| TT | 4 | 4 | – | – | – | – |
| BL | 1 | – | 1 | – | – | – |
| LL (including 4 cases of histoid leprosy) | 7 | – | – | – | 6 | 1 |
| Total | 12 | 12 | ||||
| Histopathological diagnosis | Slit-skin smear cytological diagnosis (48 cases) | ||||
|---|---|---|---|---|---|
| Type | No. of cases | Tuberculoid (BT and TT) | BL | Nonspecific inflammation | Inadequate material |
| TT | 6 | 6 | – | – | – |
| BT | 17 | 15 | – | – | 2 |
| BL | 2 | – | 2 | – | – |
| Indeterminate leprosy | 12 | 0 | – | 6 | 6 |
| Total | 37 | 37 | |||
As depicted in Tables 3 and 4, cytohistological correlation was achieved in 33 of 49 cases. It was found that both the polar ends of the spectrum (lepromatous and tuberculoid types) showed 100% correlation, while indeterminate leprosy showed no correlation at all. The cases that were diagnosed histologically as borderline lepromatous type showed 66% correlation (2 of 3 cases) in cytology.
As depicted in Table 5, the overall diagnostic accuracy of fine-needle aspiration smears was 83.3% and that of slit-skin smears was 62.1%.
| Technique | Total cases | Cases with adequate material | Histologically correlated | Diagnostic accuracy | |
|---|---|---|---|---|---|
| On adequate material | Overall | ||||
| FNA Slit-skin smear |
12 37 |
11 29 |
10 23 |
10/11 (90.9%) 23/29 (100%) |
10/12 (83.3%) 23/37 (62.1%) |
| Overall cytology | 49 | 40 | 33 | 33/40 (82.5%) | 33/49 (67.3%) |
As depicted in Table 6, 12 cases were positive for acid– fast bacilli in tissue sections (modified Fite stain) and 9 cases were positive in skin smears (ZN stain).
| Histopathological diagnosis | No. of cases | Tissue section positivity | Skin smears | |
|---|---|---|---|---|
| Positivity | Inadequate sampling | |||
| TT | 10 | 0 | 0 | 0 |
| BT | 17 | 2 | 0 | 2 |
| BB | 0 | 0 | 0 | 0 |
| BL | 3 | 3 | 3 | 0 |
| LL | 7 | 7 | 6 | 1 |
| Indeterminate leprosy | 12 | 0 | 0 | 6 |
| Total | 37 | 12 | 9 | 9 |
Figure 1 to 4 depict the various lesions that were observed in the present study.
![Tuberculoid leprosy. (a) Raised erythematous plaque on the cheek. (b) Microphotograph showing well-formed granulomas (May–Grunwald–Giemsa [MGG] stained, 40x).](/content/164/2024/16/2/img/JLP-16-170-g001.png)
- Tuberculoid leprosy. (a) Raised erythematous plaque on the cheek. (b) Microphotograph showing well-formed granulomas (May–Grunwald–Giemsa [MGG] stained, 40x).

- Borderline tuberculoid leprosy. (a) Hypopigmented patches on the back. (b) Hematoxylin and eosin (H&E) stained section(10X magnification) showing irregular thinned out grenz zone, with prominent granulomas, l ymphocytes, and Langhan’s type of giant cells. (c) Corresponding May– Grunwald–Giemsa (MGG) stained cytosmears (40X magnification) showing well-formed granulomas with epithelioid macrophages and lymphocytes.

- Lepromatous leprosy. (a) Nodular skin lesions over the back. (b) Hematoxylin and eosin (H&E) stained section (10X magnification) showing prominent grenz zone with sheets of foamy macrophages with inset showing Fite stained section (40X) with bacteriological index of 5 to 6þ. (c) Corresponding May–Grunwald–Giemsa (MGG) stained smear showing plenty of foamy macrophages in small clusters (40X magnification) with inset showing modified Ziehl–Neelsen (ZN) stained smears with bacteriological index of 5 to 6þ (40X magnification).
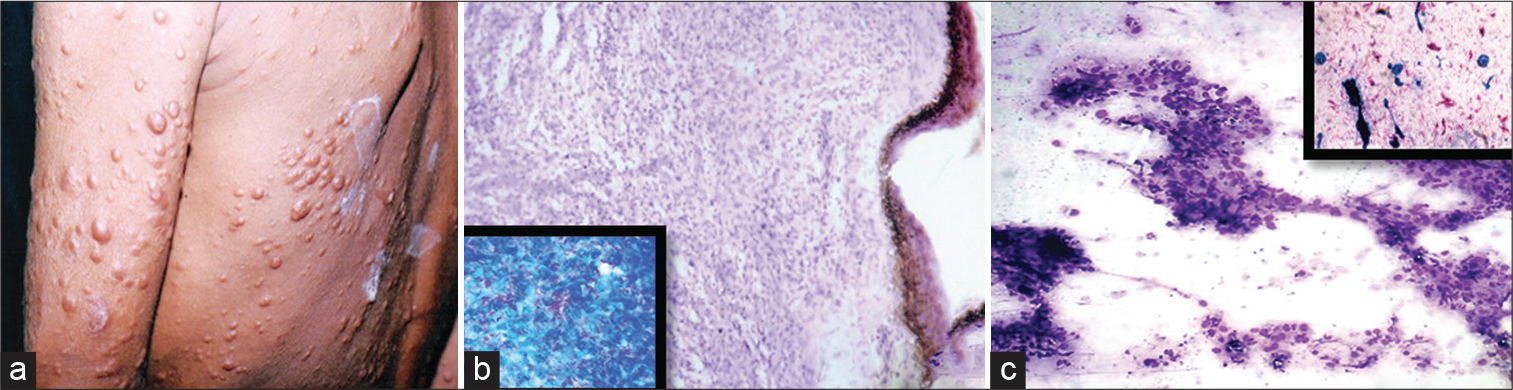
- Histoid leprosy. (a) Multiple large nodular lesions. (B) Hematoxylin and eosin (H&E) stained section (10X magnification) showing interlacing bundles of spindle-shaped histiocytes in the dermis with inset showing Fite-stained section showing a very high bacterial index (40X magnification). (c) Corresponding May–Grunwald–Giemsa (MGG) stained cytosmear (10X magnification) showing spindle-shaped macrophages in whorls and interlacing bundled with inset showing modified Ziehl– Neelsen (ZN) stained smear (10X magnification) with high bacteriological index.
DISCUSSION
The RJ scale divides leprosy into five histologically recognizable groups based on immunological spectrum. Although histopathology has been the gold standard in diagnosing and accurately typing the lesions, it has been opined[18] previously that cytological study of the cellular exudates can not only help in detecting bacteriological and morphological indices but also be used to place the case in approximate position on the modified RJ scale (three-tier system). Cytological methods being much easier, less time-consuming, and fairly accurate, the first attempt to classify leprosy with the help of exudate was made by Ridley et al[18] as early as 1989.
The slit-skin and fine-needle aspiration techniques were used to obtain cytological material based on the type of lesion. Sample inadequacy was more common in the former technique. Our study results are similar to that obtained by Ray et al[19] who presented that fine-needle aspiration technique could be performed in any kind of lesion and was superior as it was free from confounding epidermal squamous cells and better suited for cytological examination.[20]
In the present study, it was identified that specimen inadequacy was most common in the cases which showed features of indeterminate leprosy changes on histology (6 of 9 inadequate samples). The reason for this could be incorrect selection of sampling site, as it was performed by pathologists in the present study or the familiar immunological instability of patients with leprosy, who can present with different kinds of lesions concurrently.[21] Ray et al[19] in their study showed that the diagnostic accuracy can be increased by performing aspirations from multiple sites in a single patient.
In the present study, the overall concordance between cytology and histology was 76.7%. Our results are similar to that obtained by Sehgal and Joginder[22] and slightly higher than the study by Jaswal et al.[23] The polar forms of leprosy (tuberculoid and lepromatous forms) showed 100% concordance when the sampling was adequate. These results are similar to that obtained by various authors.[10,11,23] In the present study, it was not possible to differentiate BT and TT Hansen’s accurately using cytological criteria alone. Our findings are similar to that obtained by others[10,11] who suggested that it was simpler to use a three-tier classification on cytology (tuberculoid, midborderline, and lepromatous) for better cytohistological concordance. However, BL and LL Hansen’s were accurately differentiated on cytology in the present study. The most useful criterion here was the presence of aggregates of macrophages mixed with numerous lymphocyte BL cases as opposed by LL cases (few lymphocytes and scattered macrophages).
In the present study, it was found that out of the total 27 cases of tuberculoid and borderline tuberculoid on histopathology, 4 patients had more than 5 hypopigmented patches and out of the 10 cases diagnosed as lepromatous and borderline lepromatous on histopathology, 1 case had 4 hypopigmented patches and another case had 3 hypopigmented patches clinically. Thus, it is opined that just using the WHO criteria for treating leprosy may result in overtreatment of a paucibacillary case and undertreatment of a multibacillary case. Our results are in concordance with those obtained by Ray et al.[19]
In the present study, 12 cases were diagnosed as indeterminate leprosy on histopathology. Of these cases, eight cases had more than five hypopigmented patches, clear sensory neural loss, and thickened nerves, and hence were clinically treated as a case of multibacillary leprosy and were given three drug regimens for a duration of 12 months as indicated by the WHO. The remaining four cases had less than one to three hypopigmented and hypoaesthetic patches with no other symptoms and were treated as paucibacillary leprosy.
CONCLUSIONS
Diagnosing leprosy in resource-limited settings is a challenge. Most centers use the WHO criteria presently for diagnosis of leprosy, which has its own limitation. By just using the WHO criteria, we may over- or under diagnose leprosy cases, which can result in over- or undertreatment. In clinical practice—if we incorporate cytological examination along with the WHO criteria—it may be possible to enhance the diagnostic accuracy of leprosy, especially in polar and stable forms of leprosy. Cytological examination, being a simple, less time-consuming, and easy technique, correlates well with the gold standard histopathology as seen in the present study.
Conflict of Interest
None declared.
Funding
None.
References
- The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical and immunological features of leprosy. Br Med Bull. 2006;77-78:103-121.
- [CrossRef] [PubMed] [Google Scholar]
- Government of IndiaAnnual Report 2021-2022, National Leprosy Eradication Programme. New Delhi: Department of Health and Family Welfare, Ministry of Health and Family Welfare, Government of India; 2022. p. :147-150.
- Leprosy elimination: not as straightforward as it seemed. Public Health Rep. 2008;123:213-216.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy: pathology In: Valia RG, Valia AR, eds. Textbook and Atlas of Dermatology (1st ed). Bombay: Bhalani Publishing House; 1994. p. :1340-1349.
- [Google Scholar]
- The use of histopathology in leprosy diagnosis and research. Lepr Rev. 1989;60:257-262.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico histopathological correlation in leprosy. Der-matol Online J. 2012;18:2.
- [CrossRef] [Google Scholar]
- A comparative evaluation of bacteriologic and morphologic indices of Mycobacterium leprae in skin, lymph node, bone marrow, nerve and muscle. Int J Lepr Other Mycobact Dis. 1975;43:55-57.
- [Google Scholar]
- Fine needle aspiration of lymph nodes in leprosy. A study of bacteriologic and morphologic indices. Int J Lepr Other Mycobact Dis. 1977;45:369-372.
- [Google Scholar]
- Cytomorphology of leprosy across the Ridley-Jopling spectrum. Acta Cytol. 1996;40:719-723.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of fine-needle aspiration cytology in the classification of leprosy. Diagn Cytopathol. 2001;24:317-321.
- [CrossRef] [PubMed] [Google Scholar]
- Leprosy: a review of epidemiology, clinical diagnosis, and management. J Trop Med. 2022;2022:8652062.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue Processing: Theory and Practice of Histological Techniques. Beijing: Elsevier; 2019
- [CrossRef] [PubMed] [Google Scholar]
- Skin Biopsy on Leprosy: Histological Interpretation and Clinical Application (2nd ed). Basel, Switzerland: CIBA-GEIGY; 1985.
- [Google Scholar]
- Classification of leprosy according to immunity: a fine group system. Int J Lepr. 1989;34:255-273.
- [Google Scholar]
- Fine needle aspiration cytology in leprosy. Indian J Dermatol Venereol Leprol. 2008;74:352-356.
- [CrossRef] [PubMed] [Google Scholar]
- The cellular exudate: Mycobacterium leprae relationship and the critical reading of skin smears. Lepr Rev. 1989;60:229-240.
- [CrossRef] [Google Scholar]
- Benefits and limitations of fine needle aspiration cytology in the diagnosis and classification of leprosy in primary and secondary healthcare settings. Cytopathology. 2015;26:238-243.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology in reactional and non-reactional leprosy. Indian J Der-matol Venereol Leprol. 2007;73:247-249.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional variations from lepromatous to tuberculoid histology: a case report. Int J Lepr Other Mycobact Dis. 1991;59:116-119.
- [Google Scholar]
- Evaluation of leprosy lesions by skin smear cytology in comparison to histopa-thology. Indian J Pathol Microbiol. 2001;44:277-281.
- [Google Scholar]






