Translate this page into:
A rare case of nodular fasciitis presenting as a parotid tumor: Clues to cytodiagnosis
*Corresponding author: Seetu Palo, MD, Department of Pathology and Laboratory Medicine, Administrative Block, All India Institute of Medical Sciences, Bibinagar, Hyderabad Metropolitan Region, Telangana 508126, India seetu.pearl@gmail.com
How to cite this article: Palo S, Gargade CB. A rare case of nodular fasciitis presenting as a parotid tumor: Clues to cytodiagnosis. J Lab Physicians. 2024;16:124-9. doi: 10.1055/s-0043-1771242
Abstract
A 14-year-old boy presented with left preauricular painless swelling of 10 months’ duration. Local examination revealed a 3 × 2-cm, firm, nodular, nonmobile mass in the left preauricular area, just in front of tragus. Fine needle aspiration yielded paucicellular smears comprising singly scattered histiocyte/myofibroblast-like spindle cells and occasional giant cells. It was reported as benign mesenchymal lesion on cytology. Left superficial parotidectomy was performed. Histopathological and immunohistochemical analysis confirmed the diagnosis of nodular fasciitis (NF). It was an illcircumscribed cellular neoplasm, abutting and focally infiltrating into otherwise normal parotid gland. The tumor comprised spindle cells arranged in short bundles and storiform pattern with interspersed osteoclast-like giant cells, foam cells, extravasated red blood cells (RBCs), and focal areas of keloidal collagenization. The cells were positive for smooth muscle actin and negative for CD34, beta-catenin, S100, and pancytokeratin. Final diagnosis of NF was rendered. NF can rarely involve the parotid gland or present as parotid enlargement and pose diagnostic challenge, especially on cytology. However, in appropriate clinical context, subtle cytomorphological clues such as presence of myofibroblasts and fibrocollagenous stromal fragments can help in ruling out the commonly occurring salivary gland neoplasms and offering a definitive cytodiagnosis of NF which will be helpful in deciding the further course of management.
Keywords
nodular fasciitis
parotid
myofibroblast
cytology
INTRODUCTION
Nodular fasciitis (NF) is a benign, self-limiting, fibroblastic/myofibroblastic tumor, commonly encountered during third and fourth decades of life.[1] NF is a great masquerader and can be easily misdiagnosed clinically as sarcoma because of its presentation as a rapidly growing mass. Accurate characterization of the lesion is often not possible by radiological modalities such as ultrasonography (USG), computed tomography (CT) scan, and magnetic resonance imaging (MRI).[2] On fine needle aspiration cytology (FNAC), another very important modality of preoperative diagnosis, offering a confident diagnosis of NF can be quiet challenging. Here, we report an interesting case of NF presenting as a parotid mass in a teenager with emphasis on cytomorphological features.
CASE REPORT
A 14-year-old boy presented to the otorhinolaryngology outpatient department with the complaints of painless, left preauricular swelling of 10 months’ duration. There was no history suggestive of chronic sialadenitis/toothache/trauma to the site. Local examination revealed a 3 × 2-cm, ill-defined, firm, nodular, nonmobile mass in the left preauricular area, just in front of tragus. There was no facial nerve weakness or other intraoral lesions or cervical lymphadenopathy. FNAC yielded paucicellular smears comprising singly scattered histiocyte/myofibroblast-like spindle cells and rare multinucleated giant cells (Figure 1a–c). Occasional collagenous fragments were also noted (Figure 1d). No ductal cells or acinar cells were found. A diagnosis of benign mesenchymal neoplasm was rendered. Subsequently, the patient underwent left superficial parotidectomy by external approach using modified Blair incision (Figure 1e). Intraoperatively, a multilobulated tumor mass was found attached to the superficial lobe of parotid and fibrocartilaginous framework of preauricular cartilage. The lesion was completely excised and measured 2.4 × 1.8 × 1.2 cm in toto (Figure 1f). Histopathology was suggestive of NF (Figure 2a–i). Microscopic examination showed an illcircumscribed cellular neoplasm, abutting and focally infiltrating into an otherwise normal parotid gland. The tumor comprised spindle cells arranged in short bundles and storiformpattern with interspersed multinucleated giant cells. The lesional cells were oval to spindle with bland nucleus, fine chromatin, inconspicuous nucleoli, and ill-defined cyto-plasmic borders. Few mitotic figures were noted. Also noted were foam cells clusters, plenty of extravasated RBCs, and focal areas of keloidal collagenization. On immunohistochemistry, the cells were positive for smooth muscle actin (SMA) and negative for S100, CD34, beta-catenin, and pancytokeratin. The postoperative period was uneventful, and no local recurrence was noted at 18-month’s follow-up.
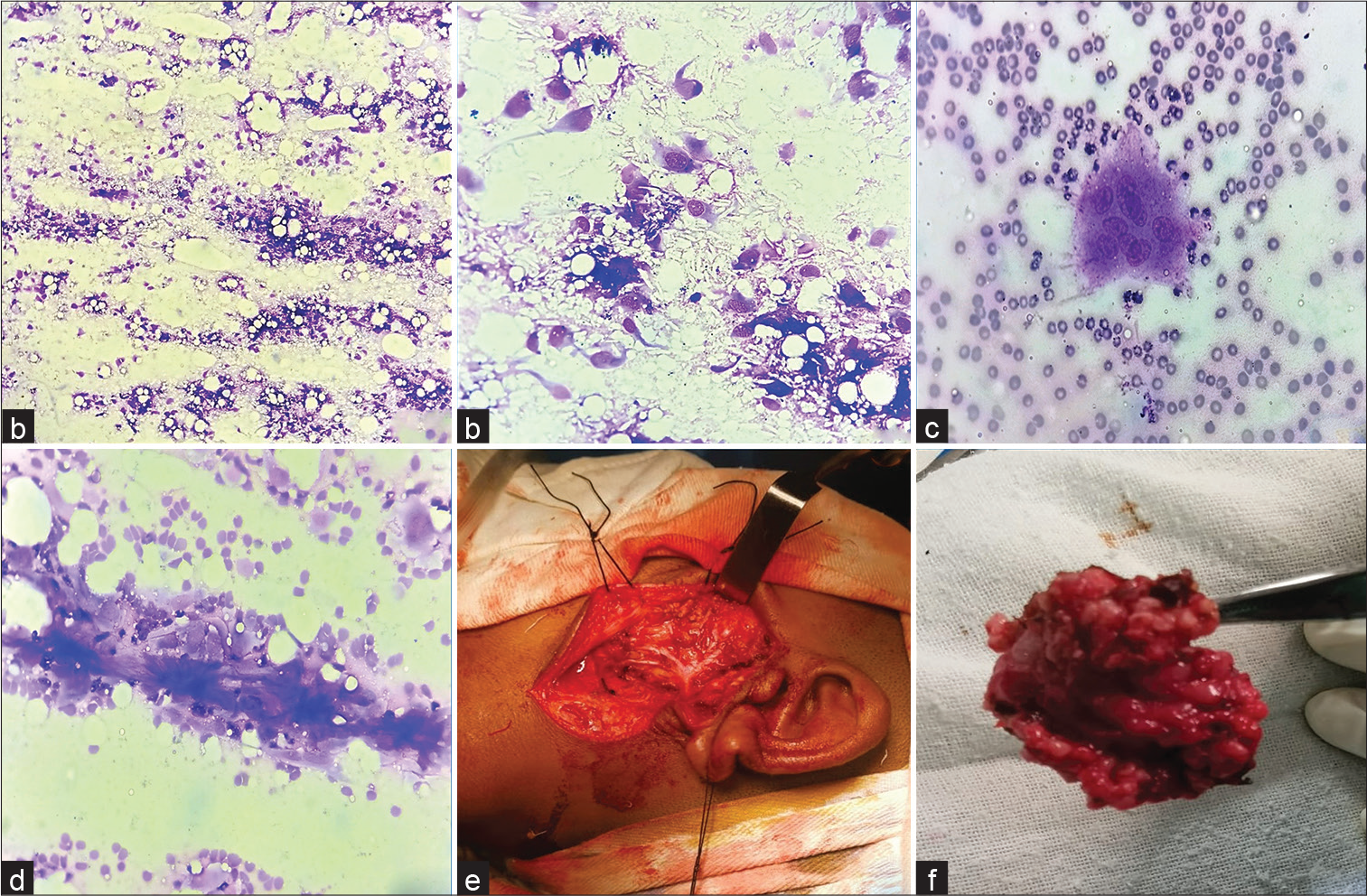
- (a) FNAC smear shows singly scattered oval to spindle cells against a haemorrhagic background (May Grunwald–Giemsa stain, ×40); (b) higher magnification showing the histiocyte/myofibroblast-like nature of the lesional cells with wispy amphophilic cytoplasm (May Grunwald– Giemsa stain, ×400); (c) a multinucleated giant cell on cytosmear (May Grunwald–Giemsa stain, ×400); (d) collagenous extracellular material/fragment (May Grunwald–Giemsa stain, ×400); (e) left superficial parotidectomy by external approach using modified Blair incision; (f) excised tumor along with superficial lobe of left parotid gland. FNAC, fine needle aspiration cytology.
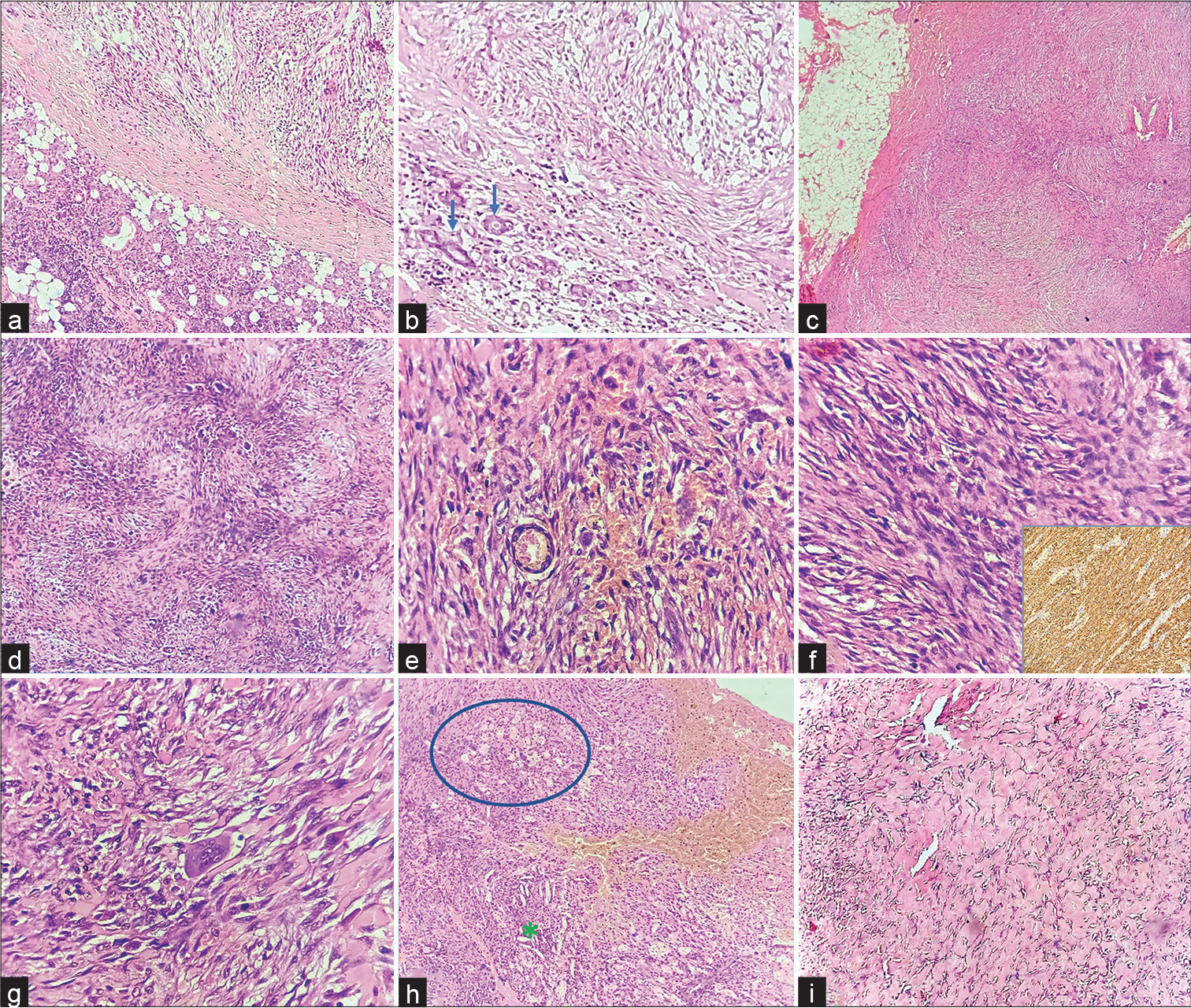
- Histomorphological features: (a) Tumor (right top) seen in juxta position to the parotid gland (left bottom) (H&E, ×40); (b) tumor focally infiltrating into the parotid parenchyma. Blue arrows point to the entrapped glandular epithelium of parotid by tumor (H&E, ×40); (c) the tumor– soft tissue interface. The surgical margins were free of tumor (H&E, ×40); (d) tumor displaying tissue culture–like appearance and storiform arrangement of cells (H&E, ×40); (e, f) oval to spindle tumor cells with bland nucleus, fine chromatin, inconspicuous nucleoli, and ill-defined cytoplasmic borders. Inset shows diffuse smooth muscle actin positivity on immunohistochemistry (H&E, ×400); (g) an interspersed multinucleated giant cell (center) (H&E, ×400); (h) a focus depicting cluster of foam cells (circled area), few cholesterol clefts (asterisk), and plenty of extravasated RBCs (H&E, ×40); (i) a focus showing keloidal collagenization (H&E stain, ×40). H&E, hematoxylin–eosin.
DISCUSSION
NF usually presents as a painful/tender, subcutaneous lesion with rapid growth pattern.[1] It can potentially occur any-where in the body but most commonly seen in upper extremities, trunk and head and neck region.[1,3] NF presenting as a parotid mass is a rarity, with only a handful of case reports and case series on the record.[4] Gibson et al analyzed 30 cases of parotid gland NF and found that male-to-female ratio was 1:1, mean age at presentation was 35.7 years, and the duration of symptoms ranged from 0.2 to 12 months.[4] They also noted that right parotid was involved more than left (right:left = 19:6) with superficial lobe being commonly affected. The size of the lesion ranged from 0.7 to 6 cm. The clinical parameters of the current case fairly corroborate with the findings of Gibson et al.
Spontaneous resolution of NF is a commonly noted phenomenon, and therefore, arriving at an accurate preoperative diagnosis is crucial in order to avoid surgical excision.[5,6] It should also be distinguished from its clinicopathological mimickers that occur in the parotid region. In this context, imaging tools such as USG, CT scan, and MRI lack specificity in picking up NF. NF usually displays infiltrative borders and thus can be misinterpreted as a malignant lesion radiologically.[2] As a preoperative diagnostic modality, FNAC thus lays an important role in narrowing down the clinical differential diagnoses and ruling out certain salivary gland lesions. Wong and Di demonstrated a diagnostic accuracy of 88% for rendering a diagnosis of NF on FNAC.[6]
The cytosmears of NF display predominant population of singly scattered or loosely cohesive sheets of spindle cellsadmixed with variable amount of myxoid stroma. Inflammatory cells, polygonal cells, myofibroblasts, ganglion cell-like cells, multinucleated giant cells, and fibrocollagenous stromal fragments can be present.[2–4] Mild nuclear atypia and few typical mitotic figures may be encountered, and their presence should not raise an alarm.[2,4,5] Few investigators have observed the presence of ganglion cell-like cells to be specific for the cytodiagnosis of NF.[3,6] A ganglion cell-like cell is a large polyhedral or triangular cell (at least twice the size of a plump fibroblast) with a round to oval nuclei displaying finely dispersed chromatin, prominent nucleoli, and smooth nuclear membrane.[6]
On cytology, NF presenting as a parotid mass may be misdiagnosed as the commonly encountered pleomorphic adenoma (PA) due to the presence of polygonal cells and myofibroblasts mimicking plasmacytoid myoepithelial cells and fibrocollagenous stromal fragments mimicking chondromyxoid substance as in the present case.[5] In the present case, a diagnosis of mesenchymal tumor was favored over PA due to clear-cut myofibroblast-like appearance of the spindle cells with basophilic to amphophilic cytoplasm with unipolar and bipolar cytoplasmic extensions. The occasional stromal fragment encountered displayed some degree of metachromatic hue but was clearly more opaque and coarsely fibrillary in nature as opposed to the chondromyxoid substance seen in PA. However, a confident cytodiagnosis of NF could not be made in this case owing to paucicellularity, and superficial parotidectomy was performed. Table 1 provides a comparison of cytomorphological features of present case with reported literature.
| Present case | Allison et al2 | Wong and Di6 | |
|---|---|---|---|
| Number of cases studied | 1 | 15 | 46 |
| Clinical parameters | |||
| Location of the lesion | Parotid | Parotid (n = 15) | Upper extremity (n = 31), lower extremity (n = 10), trunk (n = 3), neck (n = 2) |
| Male:female | Male | 1:2 | 17:29 |
| Median age, y (range) | 14 | 37 (11–64) | 38 (7–94) |
| Average time from symptom onset to clinical presentation (range) | 10 mo | 3.67 mo (1–8 mo) | 1 wk (1 d–26 wk) |
| Median size in cm (range) | 2.4 | 1.86 (3.5–1.0) | 1.5 (0.5–5) |
| Cytomorphological features | |||
| Cell arrangement | Singly dispersed | Singly dispersed (69.2%), tissue fragments (23.1%) |
Singly dispersed (78%), tissue fragments (22%) |
| Cell shape | Spindle to polyhedral | Spindle (100%), polyhedral (30.8%), ganglion cell-like cells (0%) |
Spindle (100%), ganglion cell-like cells (83%), polyhedral (82%) |
| Amount of cytoplasm | Moderate | Not specifically mentioned | Not specifically mentioned |
| Color of cytoplasm (in Giemsa-stained smears) | Pale basophilic | Not specifically mentioned | Pale basophilic |
| Cell borders | Ill defined | Not specifically mentioned | Well demarcated |
| Cytoplasmic processes | Both unipolar and bipolar long cytoplasmic processes seen in few cells |
Unipolar (76.9%), bipolar (38.5%) |
Both unipolar and bipolar long cytoplasmic processes seen in few cells |
| Nuclear shape | Round to oval | Round to oval (61.5%), elongated/spindle (84.6%), bent contours (30.8%) |
Round to oval, elongated/spindle |
| Bi-/multinucleation | Present | Present (15.4%) | Present (25%) |
| Nuclear placement | Central to eccentric | Eccentric (46.2%) | Central to eccentric |
| Nuclear membrane | Smooth | Smooth (100%) | Thin, smooth (100%) |
| Chromatin | Fine, evenly dispersed | Fine, evenly dispersed (100%) | Fine, evenly dispersed (100%) |
| Nucleoli | Inconspicuous | Inconspicuous (53.8%) | Conspicuous |
| Cytologic atypia | Absent | Minimal (46.2%) | Not specifically mentioned |
| Mitosis | Absent | Absent (100%) | Frequent, but not atypical mitosis |
| Necrosis | Absent | Absent (100%) | Absent (100%) |
| Background | Fibrocollagenous (rare fragments) |
Myxoid (76.9%), inflammatory (23.1%) |
Myxoid (28%), inflammatory |
| Others | – | Nuclear grooving (15.4%), bare nuclei (15.4%) |
Not specifically mentioned |
| Clinical outcome | No recurrence during 18-mo follow-up | Five patients (33.3%) had a recurrent lesion with an average time interval of 1.6 mo (range, 1–2.5 mo) postsurgery |
Spontaneous resolution after FNAC in 41 patients and no recurrence in 5 patients where excisional biopsy was performed |
Abbreviation: FNAC, fine needle aspiration cytology.
Histopathological examination showed typical features of NF, but immunohistochemistry for SMA, S100, CD34, pancytokeratin, and beta-catenin was performed to rule out desmoid-type fibromatosis and deep benign fibrous histiocytoma (BFH) as the management would differ (Table 2).[7–12]
| Nodular fasciitis | Desmoid-type fibromatosis | Deep benign fibrous histiocytoma | |
|---|---|---|---|
| Circumscription | Circumscribed or infiltrative, but not encapsulated | Poorly circumscribed with infiltrative margins | Well circumscribed and often have a fibrous pseudocapsule |
| Architecture/pattern | Feathery, or tissue culture-like appearance, often with S- or C-shaped fascicles or storiform pattern |
Long sweeping fascicles Can be hyper- or hypocellular |
Storiform architecture with short fascicles |
| Tumor cells | Plump, regular spindle-shaped fibroblasts/myofibro-blasts Usually lacks nuclear hyper-chromasia and atypia Mitotic figures may be plentiful but no atypical forms |
Uniform and slender fibroblasts/myofibroblasts Usually lacks nuclear hyper-chromasia or cytologic atypia Mitoses: absent or sparse | Spindle fibroblasts with plump, ovoid to elongated vesicular nuclei No nuclear pleomorphism or hyperchromasia Mitoses typically < 5 per 10 HPF but can be numerous. No atypical forms |
| Stromal component | Collagen may be increased focally and keloidal collagen bundles may be present Can show myxoid change, cystic degeneration | Myxoid change, keloidal collagen bundles, stromal hyalinization, hemangiopericytoma-like vessels may be present Peripheral lymphoid infiltrate can be seen |
Stromal hyalinization is relatively common Can show myxoid change, cystic degeneration Hemangiopericytoma -like vessels are common Peripheral lymphoid infiltrate can be seen |
| Immunohistochemistry | SMA: strong and diffusely positive CD34/beta-catenin/keratins/S100: negative | SMA: strong and diffusely positive Nuclear reactivity for betacatenin CD34/keratins/S100: negative |
SMA may be positive, only focally About 40% cases express CD34 Beta-catenin/keratins/S100: negative STAT6: negative |
| Genetic abnormality | MYH9–USP6 gene fusion as a recurrent event | Sporadic cases: CTNNB1 gene mutations Familial cases: APC gene mutations |
Rearrangements involving either PRKCB or PRKCD Clonal t(16;17) (p13.3;q21.3) was reported in a single case |
Abbreviations: HPF, high power field; SMA, smooth muscle actin; USP6, ubiquitin-specific protease 6.
Desmoid-type fibromatosis are locally aggressive tumors with high recurrence rate ranging from 19 to 77%.[13] Hence, a multimodal management approach by combining surgery with chemoradiation and endocrinal therapy is often warranted.[13] Even deep BFH can recur locally in approximately 20% of cases and rare examples of distant metastasis have been reported.[11] S100 was performed to rule out the rare possibility of myoepithelial lesions of parotid gland with spindle cell morphology and was negative in this case. Histopathology and an appropriate panel of immunostains usually confirm the diagnosis of NF, but in rare cases with atypical morphology, fluorescence in situ hybridization, reverse transcriptase–polymerase chain reaction, or next generation sequencing to look for ubiquitin-specific protease 6 (USP6) rearrangements can be undertaken. The MYH9 gene, located at 22q12.3-q13, is the commonest somatic fusion partner of the USP6 gene in NF.8
CONCLUSIONS
NF can rarely present as a parotid mass. Every effort should be made to accurately characterize the lesion on FNAC, so that tailored treatment options with conservative approach can be provided to such patients. Clues to cytodiagnosis include spindle cell–rich smears with myofibroblastic appearance, histiocytic multinucleated giant cells, and fibrocollagenous stromal fragments.
Note
Institution to which the work should be credited: Department of Pathology, Andaman & Nicobar Islands Institute of Medical Sciences, Port Blair.
Authors’ Contribution
S.P. contributed to the concept and study design, definition of intellectual content, literature search, grossing and analysis of the histopathological slides, retrieval of clinical details, manuscript preparation, and is the guarantor. C.B. G. contributed to the study design, definition of intellectual content, literature search, analysis of the histopatho-logical slides, manuscript editing and review.
Prior Presentation
The case was presented as an e-poster at Virtual TAPCON (IAPM-Tamil Nadu and Pondicherry chapter) 2021 conference, held from June 4–6, 2021.
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Source(s) of Support/Grants
None.
Conflict of Interest
None declared.
Financial Support and Sponsorship
None.
References
- Nodular fasciitis: A retrospective study of 272 cases from China with clinicopathologic and radio-logic correlation. Ann Diagn Pathol. 2015;19:180-185.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular fasciitis of the parotid gland: A challenging diagnosis on FNA. Cancer Cytopathol. 2018;126:872-880.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular Fasciitis-fine needle aspiration cytology diagnosis and its pitfalls, with review of literature. Iran J Pathol. 2019;14:76-82.
- [CrossRef] [PubMed] [Google Scholar]
- Parotid gland nodular fasciitis: A clinicopathologic series of 12 cases with a review of 18 cases from the literature. Head Neck Pathol. 2015;9:334-344.
- [CrossRef] [PubMed] [Google Scholar]
- Nodular fasciitis of the parotid gland, masquerading as pleomorphic adenoma. Korean J Pathol. 2014;48:366-370.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudosarcomatous fasciitis and myositis: Diag-nosis by fine-needle aspiration cytology. Am J Clin Pathol. 2009;132:857-865.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular benign and intermediate lesions of fibroblasts and myofibroblast In: Epstein JI. ed. Biopsy Interpretation of Soft Tissue Tumors. Philadelphia: Wolters Kluwer; 2015.
- [Google Scholar]
- Misses and near misses in diagnosing nodular fasciitis and morphologically related reactive myofibroblastic proliferations: Experience of a referral center with emphasis on frequency of USP6 gene rearrangements. Virchows Arch. 2018;473:351-360.
- [CrossRef] [PubMed] [Google Scholar]
- Morphologic spectrum of desmoid-type fibromatosis. Am J Clin Pathol. 2016;145:332-340.
- [CrossRef] [PubMed] [Google Scholar]
- CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: A study of 260 desmoid tumors and 191 potential morphologic mimics. Mod Pathol. 2012;25:1551-1558.
- [CrossRef] [PubMed] [Google Scholar]
- Deep “benign” fibrous histiocytoma: Clinicopathologic analysis of 69 cases of a rare tumor indicating occasional metastatic potential. Am J Surg Pathol. 2008;32:354-362.
- [CrossRef] [PubMed] [Google Scholar]
- Deep fibrous histiocytoma with a clonal karyotypic alteration: Molecular cytogenetic characterization of a t(16;17)(p133;q213) Cancer Genet Cytogenet. 2010;202:17-21.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative recurrence of desmoid tumors: Clinical and pathological perspectives. World J Surg Oncol. 2015;13:26.
- [CrossRef] [PubMed] [Google Scholar]






