Translate this page into:
A Rare Case of Splenic Littoral Cell Angioma in a Child
Address for correspondence: Dr. Recep Bedir, E-mail: bedirrecep@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Littoral cell angioma (LCA) is a rare, benign primary vascular neoplasm of the spleen. The tumor originates from the littoral cells lining the sinuses of the red pulp of the spleen. Preoperative distinction of this tumor from other benign or malign splenic lesions is difficult. Radiologically most cases present as multiple nodules. Definitive diagnosis can only be made histopathologically and immunohistochemically following splenectomy. This clinical situation can coexist with various malignancies and autoimmune disorders. Even though, it is mostly benign, since it has the potential to become malignant after splenectomy, long-term follow-up is required. We present an LCA case, which appeared as a solitary mass in the spleen of an 11-year-old girl with abdominal pain admitted to our hospital.
Keywords
Child
littoral cell angioma
spleen
vascular tumor
INTRODUCTION
Littoral cell angioma (LCA), first defined in 1991 by Falk et al.[1] is a rare, benign vascular tumor of the spleen. LCA originates from the littoral cells that line the sinusoids of the red pulp. LCA was expressed endothelial and histiocyte associated antigens, similar to littoral cells lining venous sinuses of normal spleen. LCAs seen median age: 50 years (range 1-77 years), female to male ratio 5:3. The splenic lesions can be in the form of small foci, large nodules or sometimes fill the entire spleen. Microscopic investigation revealed anastomosing monotonous vascular channels resembling splenic sinuses, but lined by tall endothelial cells with variable hemophagocytosis. The natural course is usually benign, most patients being asymptomatic. Some patients may be present with splenomegaly, hypersplenism associated thrombocytopenia or anemia. LCAs are almost always benign behavior, but there is a strong association between this type of tumor and various malignancies such as adenocarcinoma of the colon, liver or pancreas, and Crohn's disease. Many cases are diagnosed due to imaging features of isolated splenic masses. Ultrasound (USG) findings vary as heterogeneous echotexture without specific nodules to hyperechogenic, hypoechogenic or isoechogenic appearing lesions. Computed tomography (CT) typically shows multiple hypoattenuating lesions.[2] Since these types of tumors are very rare, they may result in misdiagnosis by pathologists and surgeons. Reports of LCAs, especially the solitary form in children are very rare.[3] Herein, we present the pathologic and radiologic findings of a solitary LCA in the spleen of an 11-year-old girl as a very rare case report in the literature.
CASE REPORT
An 11-year-old girl was admitted to hospital with a complaint of pain in the upper left quadrant of the abdomen. On physical examination of the upper left abdomen, there was minimal sensitivity with deep palpation. No pathological findings were observed in the urine analysis and abdominal radiography. The levels of serum α-fetoprotein and β-chorionic gonadotropin were within normal range. USG examination of the spleen revealed a 37 mm × 32 mm lesion with lobular contours in the medial inferior pole, which was solid in nature, with slightly isoechoic and echogenic patterns. Abdomino-pelvic magnetic resonance imaging revealed a 37-mm diameter lesion in the superior part of the anterior of the spleen, which showed T1A-hypointense, T2A-hyperintense signal variation, and prominently contrast substance up taking nodule [Figure 1]. As radiological findings were in agreement with a hemangioma, daily oral administration of propronalol 1 mg/kg was initiated. β-blocker administration was ceased on discovering by USG that the lesion remained unchanged during 3 months of follow-up and splenectomy was performed. Macroscopically examination, on the section surface of the spleen, a red-brown solitary mass of 4 cm × 3 cm × 2 cm in diameter was observed [Figure 2]. Microscopically examination, a benign tumor formed from vascular canals with blood-filled luminal anastomosis was observed. Cyst-like areas and micropapillar projections extending into the lumens of the vascular structures of the tumor were observed [Figure 3a]. The endothelial cells lining the tumor vasculature were elongated and swollen in appearance, with the lack of nuclear atypia and few mitotic changes [Figure 3b]. In immunohistochemical examination, tumor was positive for CD31 and CD68, but negative for CD34 and CD8 [Figures 4 and 5]. The Ki-67 proliferation index of the tumor was low (2%). Therefore, based on histopathological and immunohistochemical findings, the case was diagnosed as LCA.
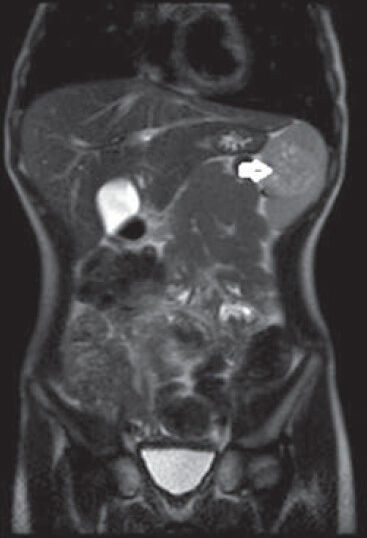
- In T2A-weighted coronal sliced sections in magnetic resonance imaging, hyperintense lesion within spleen (white arrow)
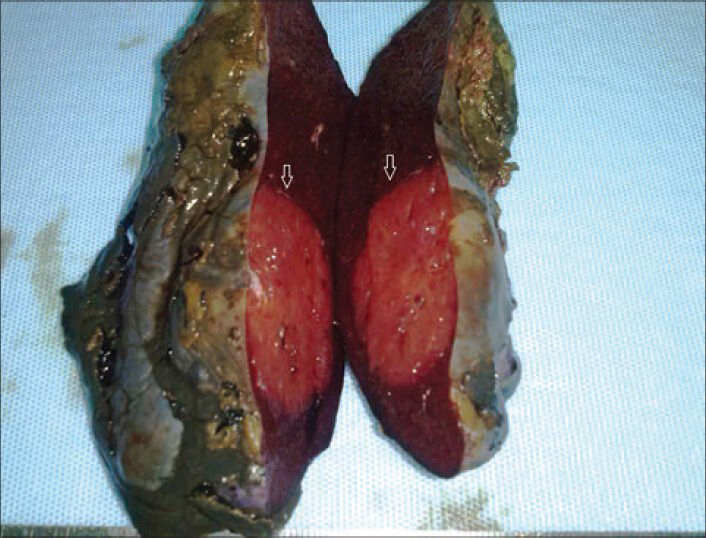
- Macroscopically examination revealed on the section surface of the spleen, a red-brown solitary mass (white arrows)
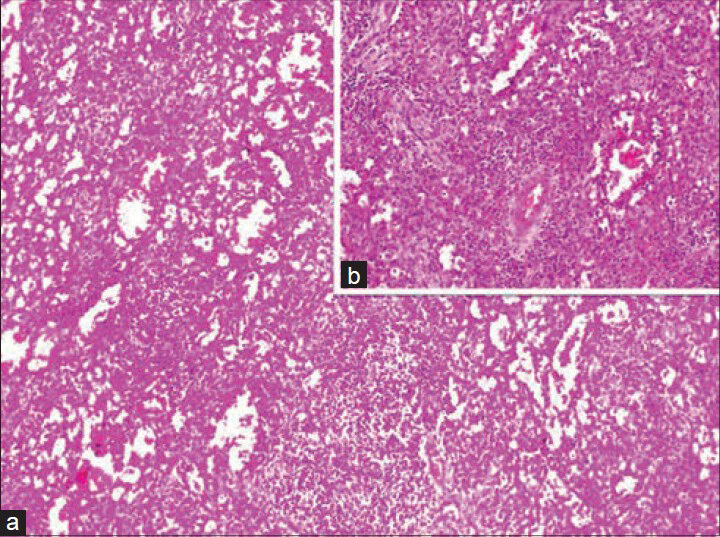
- (a) Microscopically examination revealed a benign tumor formed from vascular canals with blood-filled luminal anastomosis (H and E, ×100). (b) The endothelial cells lining the tumor vasculature were elongated and swollen in appearance, with lack of nuclear atypia and few mitotic changes (H and E, ×200)
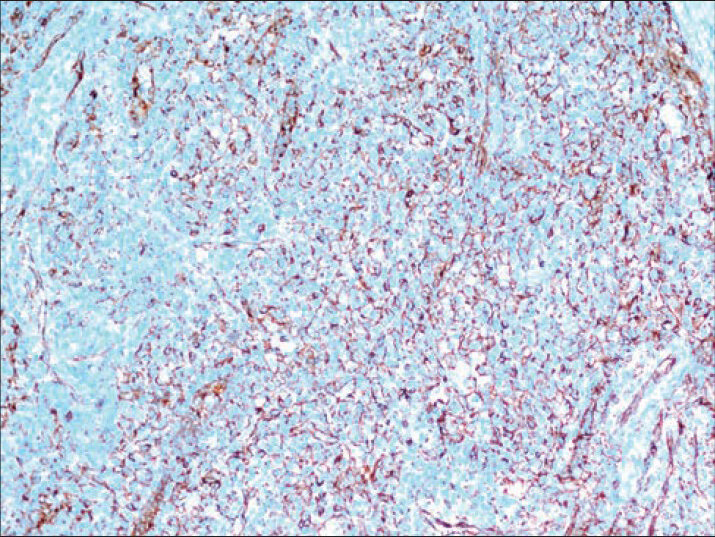
- Positive staining of endothelial cells with CD31 (Immunostaining, ×200)
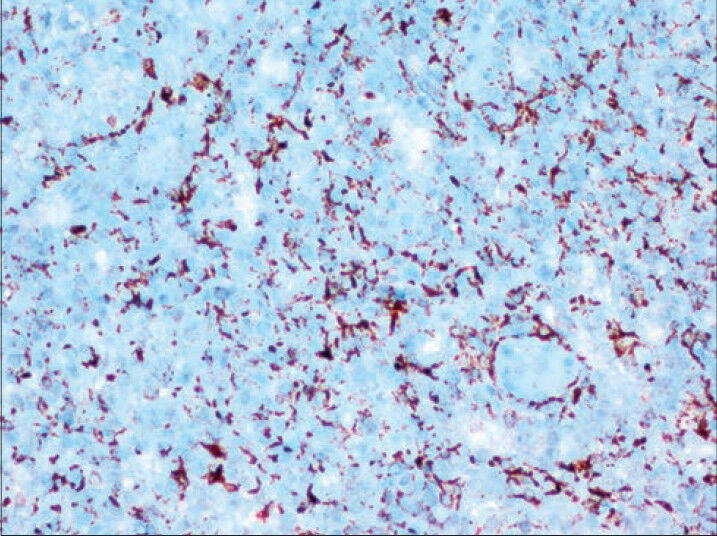
- Positive staining of endothelial cells with CD68 (Immunostaining, ×400)
DISCUSSION
The most frequently observed tumors of the spleen are vascular in origin. The majority of these are benign, hemangiomas and hamartomas. Splenic hamartomas are rare benign vascular proliferations that are discovered incidentally or during autopsy.[3] Recently, based on specific immunohistochemical properties, a new variety of splenic vascular tumor has been defined. Because of the limited number of cases and case reports, the etiology and natural course of LCA are not completely understood. About 33% of the cases reported in the literature are accompanied by various malignancies. These malignancies include lymphomas, colorectal adenocarcinomas, pulmonary adenocarcinoma, the littoral cell angiosarcoma, hemangioendothelioma, pancreatic adenocarcinoma, renal cell adenocarcinoma, melanoma, leukemia, testicular seminoma, and papillar thyroid carcinoma. In addition, 17% of the LCA cases are comorbid with immunologic or congenital abnormalities such as Crohn's disease, Wiskott-Aldrich syndrome, Epstein syndrome, lymphocytic colitis, ankylosing spondylitis, Gaucher's disease, myelodysplastic syndrome, chronic glomerulonephritis, and aplastic anemia. For this reason, there may be a link between LCA and malignancies, chronic infections or autoimmune disorders. However, this possible relation has not been exactly established because of the scarcity and lack of follow-up of LCA cases.[45] We have not come across to any clinical sign in our case indicative of malignancy, autoimmune disease, or chronic infection.
Littoral cell angiomas occur at any age and show no gender preference. Mostly, they do not present any clinical symptoms. When symptoms are present, there are several types of presentation at diagnosis: Left flank upper abdominal pain, fatigue, weakness, and weight loss. Some patients may be present with splenomegaly, abdominal pain, hyperthermia of unknown origin, and hypersplenism. Diagnosis of LCA is usually made after splenectomy for other causes. Even though, the majority of the cases of LCA in the literature are benign, several cases were with accompanying malignancies.[26]
In the differential diagnosis of LCAs, other vascular tumors of the spleen must be taken into account. Splenic vascular tumors distinct into three categories: Benign, intermediate and malign neoplasms. In the benign group are hemangiomas, lymphangiomas and hamartomas. The intermediate group comprises LCA, hemangioendothelioma and hemangiopericytoma, whilst the angiosarcomas are in the malign group. LCAs are differentiated from other tumors on the basis of their peculiar morphological and immunohistochemical properties. Lack of atypia, necrosis, and increased mitotic activity of LCA helps in distinguishing from angiosarcomas.[7] It is difficult to diagnose these tumors preoperatively because of their nonspecific clinical signs and imagings. Ramdall et al.[8] pointed out that preoperative fine needle aspiration biopsy constitutes high-risk for hemorrhagia. For this reason, definitive diagnosis of LCA can be made postoperatively based on histological and immuohistochemical characteristics. In the differential diagnosis of LCA, in addition to the elimination of the primary tumors of the spleen in the first case, granulomatous diseases such as lymphoma, visceral metastasis, abscesses of the spleen, sarcoidosis, and tuberculosis should be taken into consideration.[4]
Molecular and histological studies have shown that the endothelial cells lining the sinuses of the spleen have phagocytic and hematopoietic properties. The neoplastic cells that form LCAs stain positive for both endothelial (CD31 and factor VIII) and histiocytic (CD68, KP1, and lysozyme) markers. LCAs are negative for CD8, CD34, and S-100 markers.[789]
Imaging findings of LCAs are nonspecific. USG shows typical lobular splenomegaly and solitary or multiple echogenic nodules that may contain cystic areas. CT scanning without contrast does not show splenomegaly, well, or ill-defined margins form multiple hypodense lesions. Imaging of LCA by CT with contrast can show small multiple hypodense nodules in the portal vein phase. These images may mimic malign metastatic diseases, Kaposi's sarcoma and lymphoma or microabcesses. The majority of the vascular tumors of the spleen display delayed intensity images similar to LCA.[31011] MR scanning shows iso- and hyper-intense lesions on T1- and T2- weighted imaging's, respectively. In some cases, LCAs provide hypointense images even after gadolinium administration in both T1- and T2- weighted imagings.[41011] MR imaging characteristic of LCA appears to depend on the amount of siderosis within the tumor. If there is substantial siderosis, markedly low signal intensity deposits due to hemosiderin can be seen in all sequences. However, even though, this appearance is a very helpful and characteristic finding, it has been observed in several cases only.[4]
Littoral cell angiomas in general comprises multiple nodules that are macroscopically red-brown in color, spongy in texture, blood-filled, and lack capsule. The diameters of these nodules range from small foci to large nodules with smooth or irregular contours. As in our case, the solitary nodule form of LCA is rare and there are four case reports in the literature.[410] Microscopically, the lesions are variable anastomosed vascular structures. The endothelium lining the vascular structures forms luminal smooth, elongated, and papillar projections or cysts-like spaces. The endothelial cells are shed into the vascular lumen and show hemophagocytic properties.[10]
CONCLUSION
Littoral cell angiomas are benign vascular tumors of the spleen, which are mostly detected incidentally. However, their frequent coexistence with malignancies and the literature reports concerning their acquisition of malignant potential after splenectomy so that regular a long-term patient follow-up is warranted.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023-33.
- [Google Scholar]
- Littoral cell angioma of the spleen: A surprising cause of anemia. Rom J Morphol Embryol. 2013;54:885-8.
- [Google Scholar]
- Is littoral cell angioma of the spleen as rare as previously believed in the pediatric population? Folia Histochem Cytobiol. 2012;50:480-5.
- [Google Scholar]
- The splenic Littoral cell angioma in China: A case report and review. World J Surg Oncol. 2011;9:168.
- [Google Scholar]
- Primary vascular neoplasms unique to the spleen: Littoral cell angioma and splenic hamartoma diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2007;35:137-42.
- [Google Scholar]
- Splenic vascular tumors: A histologic, immunophenotypic, and virologic study. Am J Surg Pathol. 1997;21:827-35.
- [Google Scholar]
- Solitary littoral cell angioma of the spleen: Computed tomography and magnetic resonance imaging features. J Comput Assist Tomogr. 2008;32:772-5.
- [Google Scholar]
- Littoral cell angioma of the spleen: Report of three cases and a review of the literature. Chin Med J (Engl). 2011;124:3423-6.
- [Google Scholar]





