Translate this page into:
Alternative to xylene as a clearing agent in histopathology
Address for correspondence: Dr. Nasar Alwahaibi, Department of Allied Health Sciences, College of Medicine and Health Sciences, Sultan Qaboos University, P. O. Box 35 Postal Code 123, Muscat, Oman. E-mail: nasar@squ.edu.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Clearing is an essential step in processing tissue for light microscopy. Xylene is the clearing agent used most commonly worldwide. Xylene is toxic and therefore a threat to personnel working in histopathology laboratories. We evaluated a safer alternative clearing agent for use in the histopathology laboratory.
MATERIALS AND METHODS:
We used 230 formalin-fixed, paraffin-embedded tissue blocks from 19 different tissues. Half of the specimens were processed using xylene and half were processed using UltraClear™. Tissues were evaluated for eight parameters: sectioning, nuclear staining, cytoplasmic staining, overall cell morphology, clarity of staining, uniformity of staining, quality of immunohistochemistry (IHC), and cost.
RESULTS:
Both UltraClear™ and xylene processed sections scored 100% for IHC. Sections processed using UltraClear™ were easy to cut (81.7%) as were xylene processed sections (96.5%). UltraClear™ processed sections showed 67%, 60.9%, 52.2%, 63.5%, and 67% for nuclear staining, cytoplasmic staining, cell morphology, clarity of staining, and uniformity of staining, respectively. UltraClear™ is twice as expensive as xylene. We found that tissues processed using UltraClear™ were easy to cut and worked well for both hematoxylin and eosin and IHC staining.
CONCLUSION:
UltraClear™ is less toxic, less flammable, friendlier to the environment, and easy to handle, but it is two times expensive than xylene. The findings of this study recommend the use of UltraClear™ solution as a routine clearing agent in histopathology laboratories. However, further studies are required.
Keywords
Clearing agent
histopathology
histoprocessing
UltraClear™
xylene
Introduction
Clearing is an essential step in histopathology processing for light microscopy. The purpose of clearing is to remove dehydrating agents from tissues and to prepare the tissues for impregnation with the embedding agent. Xylene is the clearing agent used most commonly worldwide. Xylene is a sweet smelling, colorless, aromatic hydrocarbon in liquid or gas form that is found naturally in coal, petroleum, and wood tar.[1] Xylene is preferred by many histologists, because it removes alcohols from tissues rapidly, renders tissues transparent, and facilitates paraffin infiltration.[2] Xylene also is used as a deparaffinizing solvent, for coverslipping, as a solvent to clean microscopes objectives after use of synthetic immersion oil, and for recycling of used slides.[3] Xylene also is used as a solvent in the rubber, printing, and leather industries.[4]
The primary problem with using xylene is its toxicity; long-term exposure is particularly injurious to health. Inhaling xylene vapor causes skin, eye, respiratory tract, and mucous membrane irritation. Xylene also affects the central nervous system and may result in headache, weakness, memory loss, irritability, dizziness, giddiness, loss of coordination and judgment, respiratory depression or difficulty in breathing, loss of appetite, nausea, vomiting, shivering, unconsciousness, coma, and possible death due to respiratory failure. Ingestion of xylene may cause gastrointestinal irritation including abdominal pain, nausea, and vomiting. Xylene also may affect the liver and kidneys.[5]
Much has been written about the use of safer, less expensive xylene substitutes.[16] Alternative clearing agents include limonene, benzene, toluene, aliphatic and aromatic hydrocarbons, and various types of oils. Some of the alternatives, such as oil solvents, require long processing time. Others such as benzene, limonene, and toluene damage the tissue samples.[1]
UltraClear™ is a colorless, odorless isoparaffin-based liquid that contains C11–12 hydrocarbons that are derived from crude oil fractionation and cracking operations. It is environmentally friendly and exhibits no toxic effects on humans.[7] Physical and chemical properties of UltraClear™ and xylene are shown in Table 1. We compared the use of UltraClear™ versus xylene as a clearing agent in histopathology laboratory.

Materials and Methods
Preparation of tissues
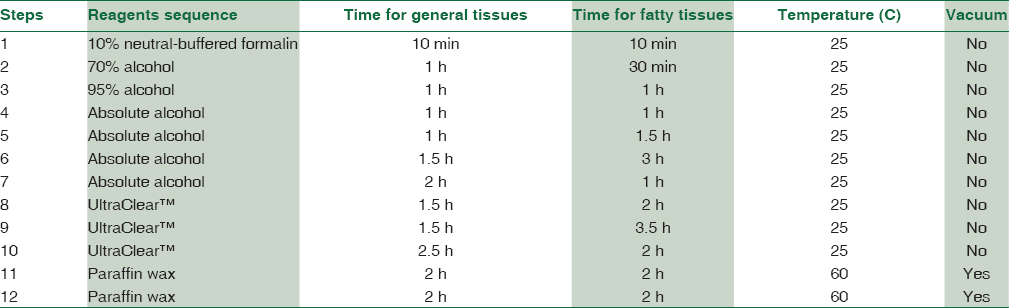
Nineteen different fresh surgical tissues were obtained and included placenta, for umbilical cord, gall bladder, prostate, appendix, ovary, spleen, products of conception, fallopian tube, breast, thyroid, sebaceous cyst, testis, lipoma, uterus, lung, kidney, omentum, small intestine, and fibroid. Each specimen was cut in half to create two groups. One group was processed using xylene (Fisher Chemical, Leicestershire, UK) and the other group was processed using UltraClear™ (Avantor's J. T. Baker, Deventer, the Netherlands). All tissues were fixed in 10% neutral buffered formalin for 24 h. All tissues were processed using an automated histoprocessor (Spin Tissue Processor Microm STP 120; Thermo Scientific, Walldorf, Germany). All tissues were dehydrated through 70%, 95%, and four baths of absolute alcohol. Half of the dehydrated tissues were cleared in three changes of xylene as follows: xylene one and two for 1 h and each and xylene three for 2 h. The other group was cleared in UltraClear™. An alternative histoprocessing program was set for particularly fatty tissues [Table 2]. All tissues were embedded in molten paraffin wax using an embedding machine (HistoStar™; Thermo Fisher Scientific) to prepare blocks. Paraffin blocks were cut at 3 μm using a rotatory microtome (Leica RM2135, Nussloch, Germany).
Hematoxylin and eosin staining
All slides were stained with hematoxylin and eosin (H and E)[8] using an autostainer (Tissue-Tek Prisma; Sakura, Nagano, Japan) to evaluate overall tissue morphology. All slides were examined by light microscopy (Olympus, BX 51, Tokyo, Japan). Nuclear and cytoplasmic staining, cell morphology, clarity of staining, and uniformity of staining criteria were used to assess the quality of all sections.[9] It is important to note that UltraClear™ was not used in all steps of staining.
Immunohistochemistry
To determine the effects of UltraClear™ on demonstration of selected antigens in tissue blocks, an equal number of slides from both groups was subjected to immunohistochemistry (IHC). Table 3 shows common antigen markers that we tested for different tissues. The Autostainer Link 48 (DAKO, Glostrup, Denmark) was used to immunostain all slides. Positive and negative controls were run with the test.

Evaluation
Each section was evaluated using modified evaluation criteria as shown in Table 4.[9] Evaluation was performed blindly by three senior biomedical scientists who work in our histopathology laboratory. The cost of UltraClear™ and xylene was obtained from local suppliers.

Results
We used 115 tissue blocks for each group. Among 115 blocks processed using UltraClear™, 81.7% were easy to cut, whereas 21 (18.3%) exhibited some difficulty during sectioning. By comparison, 111 (96.5%) xylene processed blocks were easy to cut, and 4 (3.5%) were difficult to cut. Nuclear staining in sections processed using UltraClear™ exhibited 67.0% adequacy compared to 88.7% for sections processed using xylene. Other parameters are shown in Table 5. H and E staining of testis and small intestine processed using UltraClear™ were as good as for sections processed using xylene [Figures 1 and 2]. Furthermore, sections processed using UltraClear™ exhibited immunohistochemical staining similar to that of sections processed using xylene [Figures 3 and 4]. UltraClear™ is significantly more expensive than xylene, however. One liter of xylene costs two Omani rial (OMR) which is equivalent to 5.19 US Dollar (USD) whereas of UltraClear™ costs 4.1 OMR equivalent to 10.64 USD.

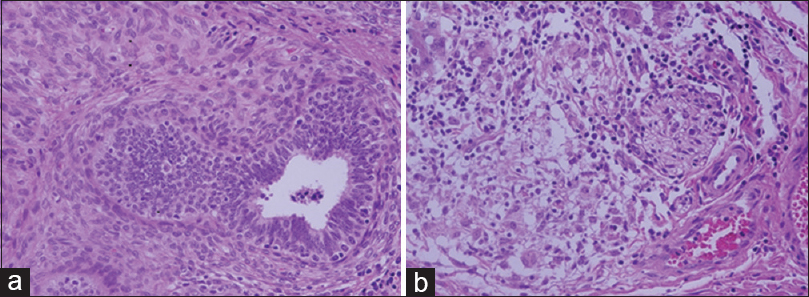
- Hematoxylin and eosin stain of testis at magnification ×40. (a) UltraClear™ processed, (b) Xylene processed

- Hematoxylin and eosin stain of small intestine at magnification ×40. (a) UltraClear™ processed, (b) Xylene processed

- Immunohistochemistry stain of gallbladder using smooth muscle actin marker at magnification ×40: (a) UltraClear™ processed, (b) Xylene processed
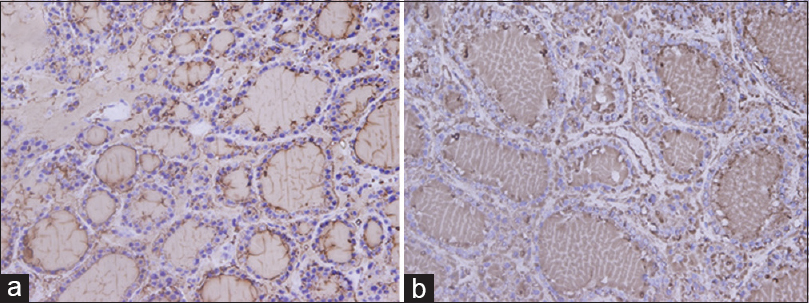
- Immunohistochemistry stain of thyroid using thyroglobulin marker at magnification ×40: (a) UltraClear™ processed, (b) Xylene processed
Discussion
We investigated the efficacy of an alternative to xylene as a safer clearing agent. Clearing is important for removing alcohols and permitting tissue infiltration with paraffin wax. Others have investigated alternative clearing agents that preserve morphology and staining characteristics of tissue sections while reducing cost. To the best of our knowledge, UltraClear™ has not yet been investigated for use as a clearing agent.
We found that immunohistochemical staining of tissues cleared with UltraClear™ was similar to those cleared with xylene. All the demonstrated antigens including vimentin, protein 63, prostate-specific antigen, pan cytokeratin, CD3, Wilms' tumor, estrogen receptor, thyroglobulin, CD34, and smooth muscle actin are clearly identified with very less background, similar to that in the routine procedure. Both clearing agents scored 100% for the quality of IHC. This finding is in line with other study which reported identical results using mineral oil as a clearing agent.[10]
Blocks of tissues processed using UltraClear™ produced good serial sections. The tissues that were difficult to section were fatty tissues such as breast and lipoma that might have been cleared incompletely, which would affect microtomy adversely. Therefore, fatty tissues may require prolonged exposure to UltraClear™ to achieve complete clearing.
We observed differences in tissue transparency, production of serial sections, and clarity of histological staining between tissues processed using UltraClear™ or xylene. Despite the fact that there is a difference in the percentages between UltraClear™ and xylene processed tissues, histologists and pathologists in our institution believe that UltraClear™ is comparable with xylene.
Others have investigated alternative clearing agents such as a novel, nontoxic xylene substitute (SBO). It was found that SBO can be used as a safe alternative for xylene. SBO processed sections were easy to cut and preserved good cell morphology after H and E staining.[11] Another study compared xylene with three clearing agents, Sub-X®, Bio-clear®, and Shandon Xylene Substitute®. These investigators concluded that their clearing agents could replace xylene for routine histopathology.[12] Similar findings were reported using n-heptane as a clearing agent.[13] Furthermore, coconut oil, which is nonhazardous, inexpensive, and causes less shrinkage of tissues was reported to be an efficient substitute for xylene.[6] Similar findings were also reported using bleached palm oil.[3] Carrot oil, olive oil, pine oil, and rose oil also can be used as clearing agents instead of xylene.[14] Groundnut oil, coconut oil, and palm kernel oil also have been recommended as alternative clearing agents for processing histological sections of wood.[15]
UltraClear™ is more expensive than xylene. There is only one sole agent that sells UltraClear™ in Oman. In addition, the use of UltraClear™ is not yet common. More important is the safety of workers in histopathology laboratories.
Our study has several limitations. First, the absence of biopsies such as gastric, liver, kidney, and skin, as they were only available for the diagnostic purpose. Second, UltraClear™ was tested as a clearing agent only, not during deparaffinization and staining.
Conclusion
We found that tissues cleared with UltraClear™ are easy to cut and produced good results for H and E and immunohistochemical staining methods. UltraClear™ is less toxic, less flammable, more friendly to the environment, and easier to handle than xylene, but it is expensive. The findings of this study recommend the use of UltraClear™ solution as a routine clearing agent in histopathology laboratories. However, further studies are required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank all staff in Pathology Department at Sultan Qaboos University Hospital, Muscat, Sultanate of Oman, for their cooperation and help in providing the specimens.
References
- Conventional xylene and xylene-free methods for routine histopathological preparation of tissue sections. Biotech Histochem. 2013;88:235-41.
- [Google Scholar]
- A comparative study to evaluate liquid dish washing soap as an alternative to xylene and alcohol in deparaffinization and hematoxylin and eosin staining. J Lab Physicians. 2014;6:84-90.
- [Google Scholar]
- Bleached palm oil as substitute for xylene in histology. J Pharm Clin Res. 2014;8:8-17.
- [Google Scholar]
- Material Safety Data Sheet (MSDS) for Xylene Fisher Scientific. 2008. Available from: https://www.fscimage.fishersci.com/msds/25150.htm
- [Google Scholar]
- Comparing the efficacy of coconut oil and xylene as a clearing agent in the histopathology laboratory. J Oral Maxillofac Pathol. 2014;18:49-53.
- [Google Scholar]
- 2010. Material Safety Data Sheet (MSDS) for UltraClear™ Avantor Performance Materials B.V. Available from: http://www.avantormaterials.com/documents/MSDS/DEV/GB/MSDS_3905_GB.pdf
- Theory and Practice of Histological Techniques (6th ed). Philadelphia, USA: Churchill Livingstone Elsevier; 2008. p. :126-7.
- Comparison of honey with ethanol as an oral cytological fixative: A pilot study. J Cytol. 2015;32:113-7.
- [Google Scholar]
- Mineral oil: The best xylene substitute for tissue processing yet? J Histotechnol. 2000;23:143-9.
- [Google Scholar]
- Anovel non-toxic xylene substitute (SBO) for histology. Afr J Tradit Complement Altern Med. 2012;9:43-9.
- [Google Scholar]
- The effect of the alternative solutions to formaldehyde and xylene on tissue processing. Indian J Pathol Microbiol. 2013;56:221-30.
- [Google Scholar]
- Replacing xylene with n-heptane for paraffin embedding. Biotech Histochem. 2012;87:464-7.
- [Google Scholar]
- Bio-friendly alternatives for xylene – Carrot oil, olive oil, pine oil, rose oil. J Clin Diagn Res. 2015;9:ZC16-8.
- [Google Scholar]





