Translate this page into:
Anti-tubercular therapy-induced maculopapular exanthema – A case series
*Corresponding author: Praveen Kumar Uppala, Department of Pharmacology, Maharajah’s College of Pharmacy, Vizianagaram, Andhra Pradesh, India. praveen.chintu32@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kandra N, Balijepalli MK P, Kandra N, Uttaravelli U, Sri Venkatesh U, Somarouthu V, et al. Anti-tubercular therapy-induced maculopapular exanthema – A case series. J Lab Physicians. 2024;16:422-5. doi: 10.25259/JLP_2_2024
Abstract
Tuberculosis (TB) is the most common, treatable and curable chronic infectious disease rampant in the developing countries. Anti-TB medications may be associated with several adverse effects including cutaneous reactions. Maculopapular exanthemas (MPE) are small cutaneous reddish macules and papules. Here we present 3 tuberculosis cases, treated with antitubercular therapy (ATT) enrolled in the outpatient department of Dermatology. Chief complaints were generalized rashes with itching and redness over the body. ATT was instantly stopped. Antihistamine, corticosteroid and topical emollients were administered to all the three patients. After 3 weeks, these patients recovered (dechallenge positive). For confirmation of ATT induced maculopapular rashes and to pin down the culprit drug, sequential rechallenge with individual ATT components was performed. Similar cutaneous eruptions were observed over the body with pyrazinamide in one case and with rifampicin in the other two cases (rechallenge positive). Symptoms of rechallenge resolved with antihistamine, steroids in 10 days. To restart ATT safely, re-challenge remains the best option and continue with alternative efficient anti-TB drugs as TB requires extensive therapy. Close, vigilant monitoring of ATT patients especially those on rifampicin and pyrazinamide must be done as these may precipitate MPE. Strict pharmacovigilance measures helps in early detection, timely management and prevent ATT induced ADRs. This is pivotal to promote safer drug utilization and help to achieve better prognostic outcomes.
Keywords
Tuberculosis
Anti-tubercular therapy
Maculopapular exanthema
Macules
Papules
Rashes
Pharmacovigilance
INTRODUCTION
The maculopapular exanthema (MPE) or rash is characterized by cutaneous erosions and lesions over the body, usually lasting from 2 to 21 days.[1] The name “maculopapular” rash originates from a combination of two words “macule” (small, flat, discolored circumscribed cutaneous lesion with diameter <1 cm) and “papule” (tiny, erythematous elevated circumscribed skin bump <1 cm in diameter). Hence, “maculopapular” rash consists of both flat and raised red skin lesions. Macules >1 centimeter diameter are taken as patches, whereas merged papules or papules > 1 cm diameter are referred to as plaques.[2] Maculopapular rashes may develop due to bacterial or viral infections- rubella, scarlet fever, human immunodeficiency virus (HIV), an allergic reaction, allergic skin conditions such as eczema, psoriasis, contact dermatitis, auto-inflammation, or sometimes due to drugs.[3] In drug-induced MPE, the rashes may develop in 4–12 days of administration of a new drug and commonly fade after one to two weeks. Pyrexia (low-grade), fatigue, and myalgia may be manifested in seven to eight days.[4] MPE is the most frequent manifestation of drug hypersensitivity,and is usually based on T-cell-mediated delayed-type hypersensitivity. A considerable proportion of MPE cases will be reactive due to an underlying infection and are not or not only drug-induced. The drugs associated with cutaneous adverse drug reactions (CADRs) from the highest risk to lowest are rifampicin (RIF) (with highest CADRs), isoniazid (INH), pyrazinamide, ethionamide, ethambutol, para-aminosalicylic acid(, and streptomycin (with least CADRs). The risk of developing an adverse drug reaction (ADR) to anti-tubercular therapy (ATT) varies from 8% to 85% in various studies.[5] The prevalence of rashes associated with ATT shows that the maculopapular rash (42.5%) is the most frequently observed type, followed by urticarial, lichenoid, drug rash with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), icterus, and exfoliative dermatitis but a total of 1.3 million people died from tuberculosis (TB) in 2022 (including 167,000 people with HIV). Worldwide, TB is the second leading infectious killer after COVID-19 (above HIV and AIDS), and ATT is the most commonly prescribed drug for TB; hence, there is a need to create more awareness among all health-care professionals while prescribing the ATT drugs. Close, vigilant monitoring of ATT patients, especially those on RIF and pyrazinamide, must be done as these may precipitate MPE.
CASE REPORT 1
A lady aged 42 years, with a confirmed diagnosis of pulmonary TB (direct sputum smear microscopy), was on ATT for the past 12 weeks. The patient visited the dermatology outpatient department having complaints of itchy rashes and reddish discoloration over the skin on both hands and shoulder [Figure 1]. Physical examination revealed generalized rashes all over the upper limbs and shoulder. As she was not on any other medications, the quick diagnosis arrived was that she had ATT-induced maculopapular rash. Immediately, ATT was withdrawn and treated with antihistamine tablet diphenhydramine 50 mg daily once for 10 days, glucocorticoid prednisolone 5 mg daily for 21 days, and topical emollients and folic acid (FA) supplementation was given to protect against tuberculosis-drug-induced liver injury (TBLI) by daily gavage of INH and RIF. After the withdrawal of the suspected culprit drug (dechallenge positive), the patient recovered in three weeks. Later, sequentially, ATT monocomponents were introduced again (rechallenge). Pyrazinamide was first reintroduced, then INH and Ethambutol, followed by RIF in the end, keeping 1 week between each drug. No signs of CADR for the first three drugs were shown. While reintroducing RIF, the patient developed a maculopapular rash with severe itching within 48 h. RIF was withheld, and this case was diagnosed as ‘“RIF induced maculopapular rash.’” Symptomatic treatment was given and the lesions resolved in 10 days. An alternative ATT regimen was prescribed, replacing RIF with tablet moxifloxacin and cycloserine, second-line ATT drugs. No ADR was reported further. This case was reported to our ADR monitoring center. This case falls under the category “certain” as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale. This case was found to be moderately severe (Level 3), and the preventability analysis showed that the ADR is “not preventable” when assessed by the Modified Hartwig and Siegel scale of preventability and severity of ADR.
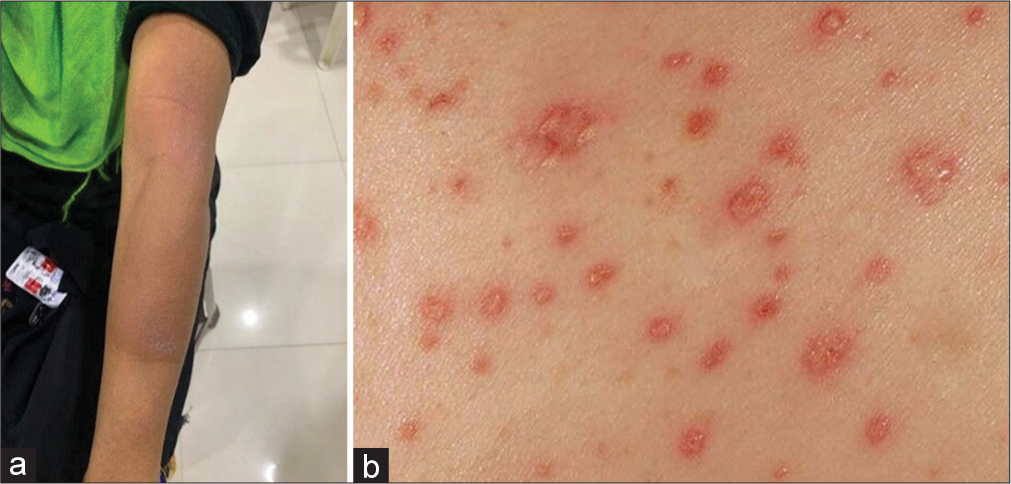
- (a) Rifampicin induced Maculopapular rash (b) View of rash.
CASE REPORT 2
A young woman aged 49 years, with pulmonary TB, was under the ATT regimen for the past five weeks. In the following week, she consulted a dermatologist, complaining of red rashes, intense itching, and light swelling on her face and lower limbs for a week. Erythematous maculopapular rashes on the face and legs were seen on clinical examination [Figure 2]. She had no preexisting cutaneous disorders or other relevant medical history of antitubercular drugs, which were withdrawn instantly. Systemic steroids (Hydrocortisone), antihistamines (tablet diphenhydramine), and topical emollients were given for symptomatic relief, and FA supplementation was given to protect against TBLI by daily usage of INH and RIF. Her lesions resolved after three weeks of stopping the ATT (dechallenge positive). A rechallenge was done to confirm that ATT was inducing the maculopapular rashes. Sequentially, single drugs of ATT were reintroduced at one-week intervals. When RIF was administered as a rechallenge, the patient developed erythematous maculopapular rashes all over her face within 48 h. Thus, the scrupulous drug was cornered and withdrawn. This case was finally confirmed as “RIF-induced maculopapular rash.” After appropriate treatment, the cutaneous lesions resolved in 10 days. Tablet moxifloxacin and cycloserine, second-line ATT drugs, were introduced in place of RIF. This case was classified as “certain” according to the WHO-UMC causality assessment scale and the report was escalated to the Indian Pharmacopeia Commission (IPC), Ghaziabad. This case was found to be moderately severe (Level 3), and the preventability analysis showed that the ADR is “not preventable” when assessed by the Hartwig and Siegel scale of preventability and severity of ADR.
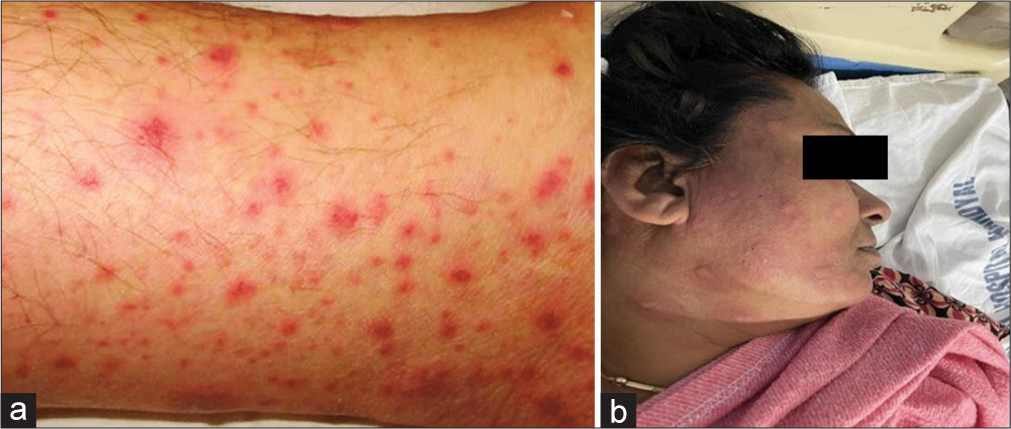
- (a) Close view of rash (b) Rifampicin induced rash.
CASE REPORT 3
A female patient aged 40 years had a persistent cough, fever, anorexia, and loss of weight for the past month. Her sputum was positive for acid-fast bacillus, confirming TB, and prescribed ATT. After two months of the intensive phase of ATT, in the third month (beginning of the continuous phase), she complained to the dermatologist of redness, and itchy rashes on both the hands and backside for one week, and eventually rashes had spread to the whole body [Figure 3]. ATT was withheld abruptly. This case was managed by administering tablet deflazacort (6 mg once daily) and tablet diphenhydramine 50 mg (antihistamine) for one week and topical mometasone (steroid) ointment and FA supplementation was given to protect against TBLI by daily administration of INH and RIF. Rechallenge with ATT drugs sequentially at one week intervals was required to confirm ATT-induced maculopapular rash. In the first week, a rechallenge was done with RIF, then in the second week, INH was reintroduced, and in the third week, ethambutol was given, and no intolerance was shown. On re-administration of pyrazinamide, the rashes reappeared and developed fever within 48 h. The miscreant drug was stopped, and the case was confirmed as a “pyrazinamide-induced maculopapular rash.” After antihistamine and steroid therapy for seven days, the patient’s symptoms resolved and was discharged. According to the WHO-UMC causality assessment scale, the case was considered as “certain” and reported from our adverse drug reaction monitoring center (AMC) to IPC, Ghaziabad. This case was found to be mild severe (Level 1), and the preventability analysis showed that the ADR is “preventable” when assessed by the Hartwig and Siegel scale of preventability and severity of ADR.
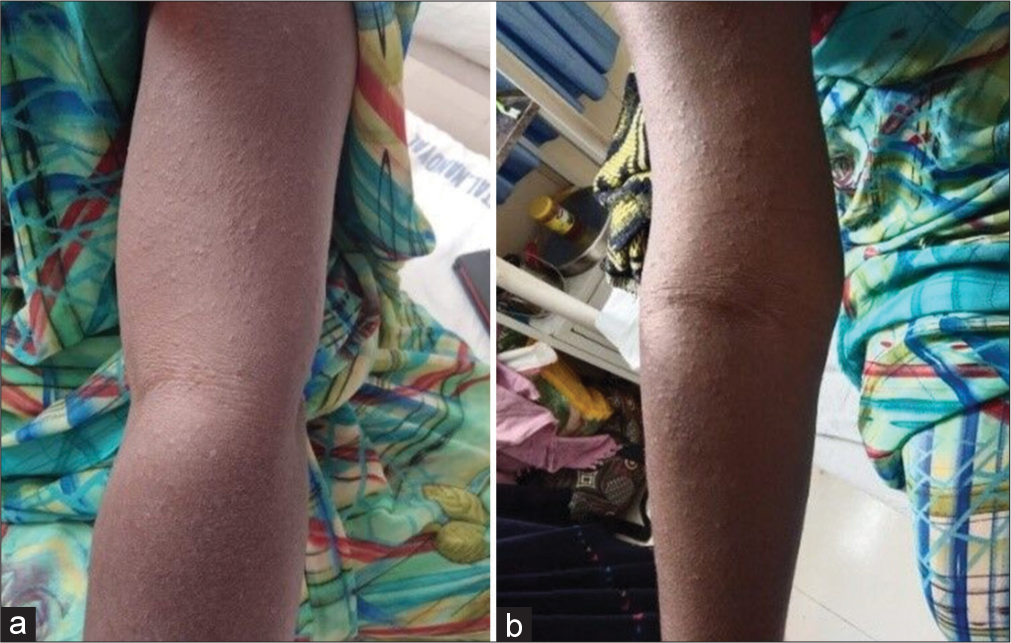
- (a) Pyrazinamide induced rash (b) Rash view
DISCUSSION
TB is the second top global infectious killer, contagious and airborne, caused by bacteria (Mycobacterium tuberculosis) primarily affecting the lungs. The first-line anti-tubercular medications include INH, pyrazinamide, ethambutol, and RIF. ATT-induced CADRs are widely reported ranging from a mild itching to fatal toxic epidermal necrolysis (TEN). MPE is one of the common dermatological presentations of delayed hypersensitivity response to drugs. Common drugs associated with MPE are penicillin, sulfonamides, phenytoin, carbamazepine, gold, etc. Since TB cases are highly prevalent in India and anti-TB drugs such as RIF and pyrazinamide are commonly used in its management, the cutaneous allergic manifestations due to RIF and pyrazinamide like MPE evoke alertness among the treating physicians.
CADR to ATT is like an ambiguous two-edged sword; on the one side, halting ATT and on the other side, initiating systemic steroid therapy may recklessly flare up the TB infection and enhance the risk of multidrug resistant TB. To identify the maleficent drug, the safest way is to sequentially re-introduce ATT monocomponents and confirm the case; only then the physician can restart a better and safer alternate anti-TB regimen.[5] CADR-related to ATT drugs such as RIF and pyrazinamide are not so well reported. If maculopapular rashes are seen in TB patients while on ATT, the clinician must suspect it as MPE-induced by RIF or pyrazinamide. In case of an ATT-induced MPE eruption, the offending agent must be immediately withdrawn and called for symptomatic management. Antihistamines, corticosteroids, and emollients are used in the general management of maculopapular rashes. Those patients under ATT may be counseled for the early identification and reporting of any cutaneous manifestations.
The etiopathogenesis of MPE involves the elucidation of proinflammatory T helper 1 mediated cytokine release and cytotoxic cluster differentiation 4 T lymphocytes.[6] Oxidative stress, various chemokines, and their receptors such as chemokine (C-C motif) ligand 20 (CCL20), CCL27, C-X-C motif chemokine ligand 9 (CXCL9), or CXCL10 are implicated in skin homing (migrate to their origin) and can worsen the MPE symptoms.[7] Exanthematous (maculopapular) drug eruption, also called morbilliform (measles-like) drug-induced exanthema, is the most common drug hypersensitivity reaction.
Carbamazepine-induced MPE is said to be associated with HLA-A*31:01 allele among the European patients,[8] HLA-B*51:01 alleles in Chinese patients,[9] and HLA-B*15:02 and HLA-B*58:01 alleles in Thai patients.[10]
ATT-induced dermatological manifestations may make the patients noncompliant, leading to treatment failure in TB therapy, and are a great dilemma to the clinicians. Prompt management of ADRs includes immediate withdrawal of the suspected drug and symptomatic treatment with antihistamines, systemic steroids, and topical emollients.
CONCLUSIONS
Before starting ATT in TB patients, screening of the risk alleles must be undertaken as it can minimize CADRs remarkably by excluding the patients with high risk. Multiple-related HLA alleles may be tested additionally as it accurately evaluates the risks of MPE eruptions in TB patients even before initiating ATT. It is crucial in any health system to monitor the safe use of drugs. As multi-drug therapy is an essential part of TB treatment that too for a prolonged period, the incidences of ADRs are unavoidable. Hence, the vital practice of reporting ADR is of utmostly appreciable as it reinforces the core evidence, thus maximizing the benefits and minimizing the risks.
Ethical approval
The research/study approved by the Institutional Review Board at Santhiram medical College and General Hospital, number IEC/SRMC/2023/148, dated July 06, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Cytokine and chemokine expression in the skin from patients with maculopapular exanthema to drugs. Allergy. 2008;63:712-9.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of maculopapular rash - Differential diagnosis of symptoms. BMJ Best Practice. Available from: https://bestpractice.bmj.com/topics/en-gb/774 [Last accessed on 2024 Dec 26]
- [Google Scholar]
- Maculopapular rash: Causes, treatment and more. Available from: https://www.healthline.com/health/skin/maculopapularrash#:~:text=A%20maculopapular%20rash%20is%20a,could%20indicate%20a%20serious%20disease [Last accessed on 2024 Dec 26]
- [Google Scholar]
- Delayed hypersensitivity reaction resulting in maculopapular-type eruption due to entecavir in the treatment of chronic hepatitis B. World J Gastrol. 2014;20:15931-6.
- [CrossRef] [PubMed] [Google Scholar]
- Spectrum of antitubercular therapy induced cutaneous adverse drug reactions and its management through rechallenge: A prospective study at a Tertiary Care Centre. Indian J Tuberc. 2022;69:470-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pyrazinamide-induced maculopapular rash. Indian J Dermatol. 2010;55:384-6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of maculopapular exanthema. Curr Opin Infect Dis. 2009;22:272-8.
- [CrossRef] [PubMed] [Google Scholar]
- HLA-A*31:01 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134-43.
- [CrossRef] [PubMed] [Google Scholar]
- Genotype-phenotype association between HLA and carbamazepine-induced hypersensitivity reactions: Strength and clinical correlations. J Dermatol Sci. 2014;73:101-9.
- [CrossRef] [PubMed] [Google Scholar]
- Association between HLA-B alleles and carbamazepine-induced maculopapular exanthema and severe cutaneous reactions in Thai patients. J Immunol Res. 2018;2018:2780272.
- [CrossRef] [PubMed] [Google Scholar]






