Translate this page into:
Antimicrobial resistance profile of Methicillin-resistant Staphylococcus aureus colonizing the anterior nares of health-care workers and outpatients attending the remotely located tertiary care hospital of North India
Address for correspondence: Dr. Pragati Grover, Department of Microbiology, GGSMC, Faridkot - 151 203, Punjab, India. E-mail: pragatigrover79@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
INTRODUCTION:
Resistance to antimicrobial agents is a major concern worldwide and is exemplified by the global spread of the Methicillin resistant Staphylococcus aureus (MRSA). Health care workers (HCWs) and asymptomatically colonized patients are important sources of nosocomial MRSA infections.
AIMS AND OBJECTIVES:
To determine the prevalence of MRSA colonisation, two hundred HCWs and 200 consecutive outpatients attending our tertiary care hospital were studied.
MATERIAL AND METHODS:
Two sterile pre-moistened cotton tipped swabs were used to collect specimens from their anterior nares. These were inoculated immediately on Blood agar with oxacillin, Mannitol salt agar with oxacillin and CHROM agar. Resistance to cefoxitin was confirmed by PCR by demonstration of mecA gene. Antibiotic susceptibility was determined by Kirby Bauer's disc diffusion method and MIC of vancomycin by using broth dilution and Vitek-2 Compact system.
RESULTS:
The nasal carriage of MRSA among HCWs was found to be 7.5% and in outpatients 3%. All strains of MRSA from HCWs and outpatients grew on three selective media and mecA gene amplified in all of them. All the isolated strains of MRSA showed high degree of resistance to co-trimoxazole (93.3%), ciprofloxacin (80%) and erythromycin (66.66%). However, there was 100% susceptiability to vancomycin, teicoplanin, linezolid and Rifampicin.
CONCLUSION:
Although a direct casual relationship could not be established, it could be assumed that the transmission from colonised health care worker is responsible atleast in part for MRSA infection among patients. Therefore emphasis should be laid on strict implementation of standard infection control practices which would help in minimizing the carriage and transmission of MRSA in the hospital.
Keywords
mecA
methicillin resistant Staphylococcus aureus
nosocomial
Introduction
There is worldwide increase in the number of infections caused by methicillin-resistant Staphylococcus aureus (MRSA) which ranges from common skin and soft tissue infections (boils, carbuncles, impetigo, cellulitis) wound infections to the more serious manifestations such as ventilator-associated pneumonias, community-acquired (CA) pneumonia, necrotizing pneumonia, necrotizing fasciitis, and sepsis.[1] It has been reported that MRSA infections occur in approximately 94,000 people each year and are associated with approximately 19,000 deaths in United States. Of these, about 86% are hospital-acquired MRSA and 14% are community-acquired MRSA (CA-MRSA).[2] MRSA is now endemic in many countries including India.[3] Clinically, its rapid emergence is posing a big problem because MRSA is not only resistant to all β-lactam antibiotics but they also express resistance to other families of antibiotics which limit the treatment options significantly. Health-care workers (HCWs) and asymptomatically colonized patients are the major sources of MRSA in the hospital environment. They constitute an important source of nosocomial infections and its dissemination both in the hospital and in the community.[4] The estimates of MRSA carriage in HCWs vary widely depending on the country, hospital specialty, and setting.[5] At present, there are a few studies on the prevalence of MRSA carriers among HCWs in the absence of an MRSA outbreak among the patients.[6] In Indian scenario, comprehensive national data on the problem of MRSA colonization are not available.[7] As there is geographical diversity in the prevalence of MRSA nasal carriage and the presence of MRSA in the hospital environment can alter the clinical outcome of the patients, the present study was undertaken to assess the nasal carriage rate and current antimicrobial profile of MRSA colonizing the HCWs and outpatients of our remotely located tertiary care hospital of Punjab. Furthermore, an attempt was made to study the MIC of vancomycin as a shift in the MIC of vancomycin within the susceptible range among MRSA strains has been reported which is associated with increasing probability of treatment failure.
Materials and Methods
A total of 200 HCWs and consecutive outpatients (200) attending our tertiary care hospital were included in the study after taking their informed written consent and permission from the Institutional Research and Ethics Committee (No. BFUHS/2k12/p-TH590 dated 16/1/2013).
Two sterile premoistened cotton tipped swabs were used to collect specimens from the each anterior nares of HCWs and the patients visiting the various outpatient departments (OPDs) of the hospital for the first time after making sure that they (OPD patients) had no contact with the HCWs and had not visited a hospital during the last 1 year. The swab was rotated five times over the inner wall of ala and nostril septum (up to a depth of 1 cm) from each nostril and were immediately (within 30 min) transferred to microbiology laboratory for inoculation on blood agar with oxacillin (BAO), mannitol salt agar with oxacillin (MSAO), and CHROMagar (HiMeReSa Media, HiMedia, Mumbai) simultaneously.[8]
All the inoculated plates were incubated at 35°C for 48 h. Colonies suggestive of MRSA on the three selective media were identified by standard techniques.[9] These were further confirmed as MRSA by studying their resistance to cefoxitin (30 mcg) using cefoxitin disc diffusion test. PCR was carried out on all MRSA strains confirmed by cefoxitin disc diffusion test for the demonstration of mecA gene using forward primer sequence of 5'-GTA GAA ATG ACT GAA CGT CCG ATA A-3'and reverse primer sequence of 5'-CCA ATT CCA CAT TGT TTC GGT CTA A-3' as described by Geha et al.[10]
Antibiotic sensitivity of the MRSA isolates was performed by Kirby-Bauer disc diffusion method on Mueller Hinton agar using antibiotic discs of erythromycin (15 mcg), gentamicin (10 mcg), netilmicin (30 mcg), co-trimoxazole (25 mcg), ciprofloxacin (5 mcg), clindamycin (2 mcg), linezolid (30 mcg), teicoplanin (30 mcg), and rifampicin (2 mcg) as per CLSI guidelines.[11] MIC of vancomycin was determined using broth dilution method and automated identification and antimicrobial susceptibility system, Vitek-2 Compact system (Biomerieux, India). S. aureus ATCC 29213 was used as a standard strain.
Statistical analysis
Chi-square test was used for statistical analysis as that P ≤ 0.05 means results are statistically significant and if P ≥ 0.05 reveals results are statistically insignificant.
Results
Of the 200 HCWs included in the study, majority [89 (44.5%)] were nurses [(working in the Intensive Care Unit [ICU] 30 (33.7%), surgery 23 (25.8%), orthopedics wards 22 (24.7%), and emergency 14 (15.7%)] followed by laboratory technicians [55 (27.5%)], doctors [40 (20%)], and ward attendants [16 (8%)]. Maximum (58.5%) of them were in the age group of 21–30 years and seventy percent (140/200) were females [Table 1].
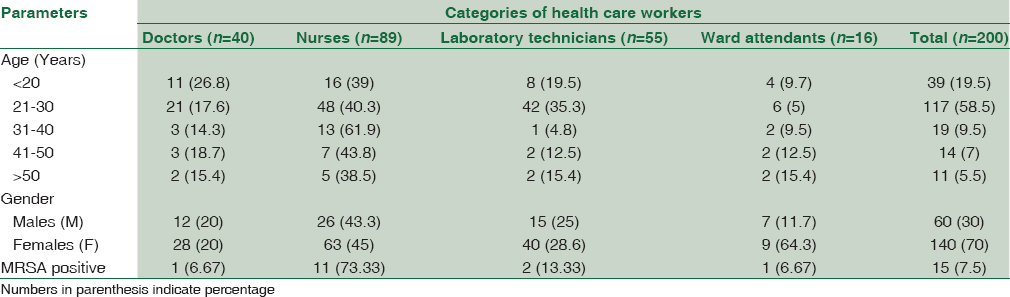
The nasal carriage rate of MRSA among HCWs was found to be 7.5% (15/200). Of these 15 MRSA strains, majority [11 (73.33%)] were colonizing nurses followed by laboratory technicians [13.33% (2/15)] and doctors and ward attendants [6.66% (1/15) each] [Table 1]. However, the differences between these groups were statistically insignificant (P > 0.05). Eight of the 15 (53.3%) MRSA isolates were obtained from HCWs of 21–30 years of age with females constituting 80% (12/15) of the total carriage.
All the 15 strains of MRSA from nasal swabs of HCWs grew on the three selective media (BAO-Blood Agar with oxacillin, MSAO-Mannitol salt agar with oxacillin, and CHROMagar-Selective media for MRSA). However, MSAO and BAO helped in rapid identification (24 h) in comparison to CHROMagar which required 48 h of incubation Moreover, mecA gene was amplified in all these 15 strains (amplicon of 310 bp). Considering PCR as the gold standard, sensitivity and specificity of all the selective media was 100%.
All the 15 MRSA strains were found to be sensitive to vancomycin, teicoplanin, linezolid, and rifampicin. Multidrug resistance was observed in the isolated MRSA strains. Maximum resistance was to co-trimoxazole (93.3%), ciprofloxacin (80%), and erythromycin (66.6%) [Table 2]. MIC against vancomycin was in the susceptible range (0.5–2ug/ml) [Table 3]. Although majority (60%) of the strains showed MIC ≤0.5ug/ml, there were 2 (13.33%) strains where the MIC was 2 ug/ml.


Among the outpatients, nasal carriage of MRSA was observed in 3% (6/200). Four of the six (66.66%) outpatients were males in the age group of 41–50 years and 2 were females in the age group of 51–60 years (33.33%). Similar to the 15 MRSA strains from HCWs, all the 6 strains of MRSA from anterior nares of outpatients also grew on the three selective media and mecA gene was amplified in all of them. Their sensitivity to vancomycin, teicoplanin, linezolid, rifampicin, and gentamicin was 100%. However, it was 66.66% to netilmicin, erythromycin, and clindamycin. There was 66.66% resistance to co-trimoxazole and ciprofloxacin [Table 2]. MIC against vancomycin was in the range of 0.5–1 ug/ml [Table 3].
There was 100% correlation between MIC of vancomycin by broth dilution method and Vitek-2 Compact system.
Discussion
Although S. aureus can colonize multiple body sites (skin, perineum, pharynx, vagina, axilla, and gastrointestinal tract) of the human beings, anterior nares of the nose is the most frequent carriage site for S. aureus.[12] The reported prevalence of nasal carriage of MRSA among HCWs in hospital settings varies between 5.8% and 17.8%.[61314] In the present study, this carriage rate was 7.5% which is comparable to studies from Turkey and Karnataka (India) from where the reported rates were 6% and 8.3%, respectively.[1415] However, studies from Nepal and another state of India (Assam) reported higher prevalence (10% and 11.43%, respectively).[1617] In contrast, low prevalence of MRSA (2.32% and 2%) has been observed in an another study from Nepal and South India, respectively.[1819] This difference could be because of the variability in the geographical areas, institutions, hospital specialties, and settings within hospital where the studies were conducted. Difference in the design of the study and methods used for detection of MRSA also accounts for the disparity in carriage rate. Some longitudinal studies have shown that the carrier state could be classified as a persistent carrier or an intermittent carrier. This is important to determine this distinction because a persistent carrier has higher bacterial load and have more chances of detection as a carrier and likewise, a known carrier may actually be an intermittent carrier.[12]
In the present study, higher proportion of MRSA carriage was observed among the nurses (73.3%) as compared to laboratory technicians, doctors, and ward attendants [Table 1] although the difference between these groups was statistically insignificant (P > 0.05). This is similar to the findings of Kalyani et al.[20] The mechanism leading to MRSA nasal carriage is multifactorial and not properly understood, but higher carriage rate in nurses poses a big epidemiological challenge because nurses are the HCWs who have the highest frequency of contact with the patients and could probably be the reservoir of infection, thus responsible for continuance of the infection in the hospital environment. The prevalence of MRSA in our study was highest in nurses working in the ICU which corroborates with the findings of Golia et al.[21] and indicates the vulnerability of the severely ill and immunocompromised patients to MRSA infections which could further complicate their treatment and chances of survival.
Majority of the MRSA carriers of our study were females which is similar to the finding of Vijaya et al.[15] In contrast, Mathanraj et al. and another review study reported male sex as an important risk factor for MRSA colonization.[91222] However, the role of gender including that of sex hormones in MRSA carriage is controversial and needs further study.
In investigations of outbreaks of infections, laboratory has a key role in identifying the colonized patients and staff. Use of highly efficient selective media is an essential part of rapid isolation of the pathogen and infection control in the hospital. Vijaya et al. used mannitol salt agar and CHROMagar simultaneously for isolation of MRSA from the anterior nares and suggested that CHROMagar should not be used as it showed low specificity (95.98%) and positive predictive value (68.49%).[15] On the other hand, Mathanraj et al. found oxacillin blood agar superior to MSAO for the isolation of MRSA.[9] However, when we compared the performance of the three selective media, they showed the same efficacy for the isolation of MRSA. However, CHROMagar required 48 h of incubation for the isolation of all the 15 MRSA strains. Thus, for the rapid isolation and identification of MRSA, BAO or MSAO was found to be equally efficacious and better than CHROMagar.
In the present study, the MRSA isolates from HCWs showed high degree of resistance to co-trimoxazole, ciprofloxacin, and erythromycin which was 93.3%, 80%, and 66.66%, respectively. Koffi et al. reported 40% resistance to co-trimoxazole, 37.5% to fluoroquinolones, and 57.8% to macrolides which confirmed their multiresistant character.[22] There was 100% susceptibility to vancomycin, teicoplanin, and linezolid in our study which is similar to the findings by Adwan et al.[23] Although MIC of vancomycin was in the susceptible range, 2 (13.33%) isolates showed increased MIC (2 ug/ml). Shashikala reported increase in MIC for vancomycin in 1.2% of the MRSA strains.[24] This warrants close monitoring of MIC of vancomycin to note a creep in its MIC which could have therapeutic implications and had shown concern over vancomycin heteroresistance in MRSA. Heteroresistant vancomycin-intermediate S. aureus (hVISA) is defined as vancomycin susceptible MRSA strain with MIC ≤2 on routine testing that upon subculture produces subcolonies with MIC in the VISA/VRSA range at the frequency ≥1 × 106 according to population analysis profile.[15] The limitation of the present study was that we could not look into the prevalence of hVISA in our MRSA isolates. The other limitation of the present study is that the sample size is relatively small and carriage rate has been investigated at a single point in time.
To ensure that the data would be applicable on national level, the studies should extend to larger population of HCWs and patients.
Asymptomatic colonized patients are another important source of MRSA in hospital environment. Nasal carriage of 3% was seen in our outpatients which is comparable to studies from India and abroad.[925] However, we are not certain whether these strains were really CA-MRSA as the molecular techniques to know the staphylococcal cassette chromosome mec type (types IV, V-CA-MRSA) or the presence of PVL toxin gene were not studied. All the 6 strains showed high degree of resistance against co-trimoxazole and ciprofloxacin (66.6% each). This is a matter of great concern as it is reflective of indiscriminate use of antimicrobial agents in a large proportion in our community.
In conclusion, MRSA carriage rate observed in the present study, though high, is in agreement with the internationally reported range of 5.8%–17.8% in the hospital settings. In this context, prevention of MRSA infection merits discussion as once introduced into hospital, MRSA spreads widely through the hands of medical personnel, colonized HCWs, asymptomatic nasal/hand carriers who act as reservoir of infection. Multiple, prolonged use of antibiotics and prolonged hospitalization are other important factors which make hospital an ideal place for transmission and perpetuation of MRSA. Therefore, emphasis should be laid on strict implementation of standard infection control practices which would help in minimizing the carriage and transmission of multidrug-resistant MRSA in the hospital. At the same time, more studies should be undertaken to identify effective barrier precautions to limit the spread of MRSA both in the hospital and in the community. Screening of health-care personnel who constitute an important infectious risk for the patients for resistant strains of staphylococcus could be adopted as a protocol to curb the spread of MRSA in the hospital and from hospital to the community. However, it would require screening of large numbers of HCWs before arriving at any definite conclusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Methicillin resistant Staphylococcus aureus (MRSA) infections. J Clin Diagn Res. 2010;4:2111-5.
- [Google Scholar]
- MRSA Causes, Symptoms, Treatment-How Common is MRSA?. Available from: http://www.emedicine health.com/mrsainfections
- Indian Network for Surveillance of Antimicrobial Resistance (INSAR) Group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence and susceptibility pattern. Indian J Med Res. 2013;137:363-9.
- [Google Scholar]
- The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, Gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136:834-44.
- [Google Scholar]
- Should healthcare workers be screened routinely for meticillin-resistant Staphylococcus aureus? A review of the evidence. J Hosp Infect. 2011;77:285-9.
- [Google Scholar]
- Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: Prevalence, duration, and transmission to households. Infect Control Hosp Epidemiol. 2004;25:114-20.
- [Google Scholar]
- Nasal carriage of meticillin-resistant Staphylococcus aureus in GPs in the West of Ireland. Br J Gen Pract. 2007;57:811-3.
- [Google Scholar]
- Vancomycin MIC creep in methicillin-resistant Staphylococcus aureus (MRSA) isolates from 2006 to 2010 in a hospital in China. Indian J Med Microbiol. 2015;33:262-6.
- [Google Scholar]
- Screening for methicillin-resistant Staphylococcus aureus carriers among patients and health care workers of a tertiary care hospital in South India. Indian J Med Microbiol. 2009;27:62-4.
- [Google Scholar]
- Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J Clin Microbiol. 1994;32:1768-72.
- [Google Scholar]
- Performance Standards for Antimicrobial Disc Diffusion Tests. Approved Standards. In: CLSI Document M2-M9 (9th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
- [Google Scholar]
- The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751-62.
- [Google Scholar]
- Nasal carriage of MRSA in GPs in the west of Ireland. Br J Gen Pract. 2007;57:811-3.
- [Google Scholar]
- Nasal carriage of methicillin-resistant Staphylococcus aureus among hospital staff and outpatients. Infect Control Hosp Epidemiol. 2004;25:169-71.
- [Google Scholar]
- Nasal carriage of MRSA in hospital personnel. J Academy of Clin Microbiol. 2011;13:71-6.
- [Google Scholar]
- Nasal carriage rate of methicillin resistant Staphylococcus aureus among at National Medical College teaching hospital, Birgunj, Nepal. Nepal Med Coll J. 2010;12:26-9.
- [Google Scholar]
- The prevalence of nasal carriage of Staphylococcus aureus among healthcare workers at a tertiary care hospital in Assam with special reference to MRSA. J Clin Diagn Res. 2013;7:257-60.
- [Google Scholar]
- Staphylococcus aureus nasal carriage among health care workers in a Nepal Hospital. Braz J Infect Dis. 2009;13:322.
- [Google Scholar]
- Nasal carriage of methicillin-resistant Staphylococcus aureus among surgical unit staff. Jpn J Infect Dis. 2009;62:228-9.
- [Google Scholar]
- Prevalence of MRSA among HCWs of Shri Satya Sai Medical College and Hospital – A tertiary care centre. J Dent Med Sci. 2012;3:23-7.
- [Google Scholar]
- A study of nasal carriage of MRSA among the health care workers of a tertiary care hospital, Bangalore. Int J Basic Appl Med Sci. 2013;3:3-7.
- [Google Scholar]
- Nasal carriage of meticillin-resistant Staphylococcus aureus among health care personnel in Abidjan (Côte d'lvoire) Dakar Med. 2004;49:70-4.
- [Google Scholar]
- Molecular analysis and susceptibility patterns of methicillin-resistant Staphylococcus aureus strains causing community-and health care-associated infections in the Northern region of Palestine. Am J Infect Control. 2013;41:195-8.
- [Google Scholar]
- Determination of vancomycin, teichoplanin and linezolid resistance among Staphylococcal isolates from a tertiary care hospital. J Acad Clin Microbiol. 2015;17:3-6.
- [Google Scholar]
- Methicillin-resistant Staphylococcus aureus infections. Top HIV Med. 2008;16:151-5.
- [Google Scholar]





