Translate this page into:
Association between Hypocalcemia and Outcome in COVID-19 Patients: A Retrospective Study
Address for correspondence: Arulselvi Subramanian, MD, Department of Laboratory Medicine, Jai Prakash Narayan Apex Trauma Centre, All India Institute Medical Sciences, Room no. 207, New Delhi, 110029, India (e-mail: arulselvi.jpnatc@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Calcium has been shown to play a vital role in the pathophysiology of severe acute respiratory syndrome-coronavirus-2 and middle east respiratory syndrome coronavirus diseases, but less is known about hypocalcemia in coronavirus disease 2019 (COVID-19) patients and its association with the disease severity and the final outcome. Therefore, this study was conducted with an aim to assess clinical features in COVID-19 patients having hypocalcemia and to observe its impact on COVID-19 disease severity and the final outcome.
Methods
In this retrospective study, consecutive COVID-19 patients of all age groups were enrolled. Demographical, clinical, and laboratory details were collected and analyzed. On the basis of albumin-corrected calcium levels, patients were classified into normocalcemic (n = 51) and hypocalcemic (n = 110) groups. Death was the primary outcome.
Results
The mean age of patients in the hypocalcemic group was significantly lower (p < 0.05). A significantly higher number of hypocalcemic patients had severe COVID-19 infection (92.73%; p < 0.01), had comorbidities (82.73%, p < 0.05), and required ventilator support (39.09%; p < 0.01) compared with normocalcemic patients. The mortality rate was significantly higher in the hypocalcemic patients (33.63%; p < 0.05). Hemoglobin (p < 0.01), hematocrit (p < 0.01), and red cell count (p < 0.01) were significantly lower with higher levels of absolute neutrophil count (ANC; p < 0.05) and neutrophil-to-lymphocyte ratio (NLR; p < 0.01) in the hypocalcemic patients.
Albumin-corrected calcium levels had a significant positive correlation with hemoglobin levels, hematocrit, red cell count, total protein, albumin, and albumin-to-globulin ratio and a significant negative correlation with ANC and NLR.
Conclusion
The disease severity, ventilator requirement, and mortality were considerably higher in hypocalcemic COVID-19 patients.
Keywords
calcium
coronavirus
mortality
neutrophil-tolymphocyte ratio
pandemic
Introduction
During the ongoing coronavirus disease 2019 (COVID-19) pandemic caused by the novel enveloped RNA β-coronavirus named severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), the world is facing unexpected challenges due to the previously unrecognized viral illness. It has been almost a year since the cluster of pneumonia of unknown etiology was found in Wuhan, China.[1] The World Health Organization (WHO) and Ministry of Health and Family Welfare, Government of India, have regularly issued diagnosis and treatment recommendations for COVID-19 disease.[2,3] The disease is still spreading rapidly around the world with a mortality rate varying from 0.7 to 10%.[4] Various studies have described the clinical characteristics and discussed the potential early and sensitive biomarkers (such as, C-reactive protein, procalcitonin, interleukin-6, ferritin, D-dimer, lymphopenia, neutrophil-to-lymphocyte ratio [NLR], etc.) to estimate the disease severity and prognosis of COVID-19 disease.[5-10] A very few studies have shown the presence of higher incidence of hypercalcemia in COVID-19 patients and a higher rate of hospitalization in those patients. Some have highlighted the association of hypocalcemia with disease severity and poor prognosis in COVID-19 disease.[11,12] Calcium has been shown to play a vital role in the pathophysiology of SARS-CoV and MERS-CoV diseases also.[13,14]
Thus, there could be a possibility that hypocalcemia in COVID-19 patients may also be associated with higher disease severity and poor outcome. However, the available information concerning this is not enough at present. Therefore, the aim of the present study was to assess the clinical features in COVID-19 patients having hypocalcemia and to determine its impact on COVID-19 disease severity and the final outcome.
Materials and Methods
This was a retrospective descriptive observational study. The study was conducted at Jai Prakash Narayan Apex Trauma Center, All India Institute of Medical Sciences, Delhi, which has become a dedicated COVID-19 care center during the pandemic. The patients are admitted here after being diagnosed positive for COVID-19 by real-time polymerase chain reaction according to the WHO guidelines. Total 161 patients of all age groups admitted during March 23 to April 30, 2020 were enrolled in the study. They were classified into mild, moderate, and severe COVID-19 disease categories according to the WHO and the Ministry of Health and Family Welfare, Government of India, guidelines.[2,3] Demographical, clinical, and laboratory details on the day of admission were collected from hospital database and analyzed thoroughly. On the basis of albumin-corrected calcium levels, patients were classified into normocalcemic (n = 51, albumin-corrected calcium level: 8.6–10.3 mg/dL), and hypocalcemic (n = 110, albumin-corrected calcium level: below 8.6 mg/dL) groups. Albumin-corrected serum calcium was calculated by the formula: albumin-corrected calcium (mg/dL) = measured total calcium (mg/dL) + 0.8 (4.0–serum albumin [g/dL]). Death was the primary outcome. The secondary outcomes were severity of disease and requirement for ventilator support. The study was conducted after obtaining the approval from the Ethics Committee of the Institute. Patients with incomplete information were excluded from the study.
Statistical Analysis
All statistical analyses were performed by GraphPad Prism 5.0 Software. Data were presented as count (percentages) for categorical variables. Results for continuous variables were expressed as mean ± standard deviation or median ± interquartile range as indicated. Categorical variables between groups were compared using the chi-square test or Fisher exact test, and continuous variables were analyzed using Student's t-test or Mann–Whitney U test as appropriate. Pearson's or Spearman's test was used to determine the correlation coefficient. A value of p less than 0.05 was considered statistically significant.
Results
Demographical and Clinical Characteristics in the Hypocalcemic Patients
Out of 161, 68.32% (110) patients were hypocalcemic. The mean age of the patients was 49.20 ± 17.07 years. Out of them, 21.82% patients were elderly and 67.27% were males. The mortality rate was 33.63%. Among the nonsurvivors hypocalcemic patients, 75.67% were males and 40.54% were elderly. Of all the hypocalcemic patients, 92.73% were classified as severe COVID-19 disease and 39.09% required ventilator support during the hospital stay. At least one comorbidity was present in 82.73% of hypocalcemic COVID-19 patients. Diabetes, hypertension, and malignancy were present in 26.36, 29.09, and 14.54% patients respectively. On multivariate analysis, calcium levels were not found to be an independent predictor of mortality (odds ratio: 1.1; confidence interval: 0.324–3.797; p-value: 0.86).
Laboratory Features in the Hypocalcemic Patients
The mean hemoglobin level was 10.31 ± 2.58 g% in hypocalcemic patients. Of all, hemoglobin level was less than 12 g% in 73.64% of the patients and less than 7 g% in 10% of the patients. The mean packed cell volume was 31.34 ± 7.76%, and the mean red cell count was 3.59 ± 0.97 million cells/μL. The median leukocyte count was 9.55 (6.8–12.3) × 103 cells/cumm, and median NLR was 2.78 (1.79–4.75). The mean total calcium was 7.67 ± 0.69 mg/dL. We observed hypoproteinaemia in 73.64% patients, with 96.36% patients having hypoalbuminemia. Aspartate transaminase was increased in 63.64% patients. Uremia was observed in 39.09% patients.
Comparison between the Normocalcemic and Hypocalcemic Patients
The details are given in ►Tables 1 and 2. The mean age of the normocalcemic patients was significantly lower than the hypocalcemic patients (43.35 ± 17.98 vs. 49.20 ± 17.07 years respectively; p < 0.05). Significantly higher number of patients (92.73%) in the hypocalcemic group were affected from severe COVID-19 disease compared with 76.47% in the normocalcemic group (p < 0.01). In comparison with the normocalcemic patient, higher number of hypocalcemic COVID-19 patients had comorbidities (82.73 vs. 64.70%, p < 0.05) and required ventilator support during treatment (39.09 vs. 15.69%, p < 0.01). The mortality rate was significantly higher (p < 0.05) in the hypocalcemic patients (33.63%) as compared with the normocalcemic patients (15.69%). Among the nonsurvivor hypocalcemic patients, 75.67% were males and 40.54% were elderly patients.
| Parameter | Normocalcemic patients (n = 51) | Hypocalcemic patients (n = 110) | p-Value |
|---|---|---|---|
| Age (years) | 43.35 ± 17.98 | 49.20 ± 17.07 | < 0.05 |
| Elderly (> 60 years of age) | 9 (17.65%) | 24 (21.82%) | 0.5419 |
| Males | 35 (68.63%) | 74 (67.27%) | 0.8642 |
| Comorbidities | 33 (64.70%) | 91 (82.73%) | < 0.05 |
| • Diabetes mellitus | 9 (17.65%) | 29 (26.36%) | 0.2256 |
| • Hypertension | 12 (23.53%) | 32 (29.09%) | 0.4613 |
| • Malignancy | 5 (9.80%) | 16 (14.54%) | 0.4624 |
| Severe patients | 39 (76.47%) | 102 (92.73%) | < 0.01 |
| Ventilator support | 8 (15.69%) | 43 (39.09%) | < 0.01 |
| Nonsurvivors | 8 (15.69%) | 37 (33.63%) | < 0.05 |
Note: Continuous data are presented as mean ± standard deviation or median ± interquartile range. Categorical data are presented as frequency (%). Bold values signify statistical significance.
| Parameter | Normocalcemic patients (n = 51) | Hypocalcemic patients (n = 110) | p-Value |
|---|---|---|---|
| Hematological parameters | |||
| Hemoglobin (g%) | 11.64 ± 2.64 | 10.31 ± 2.58 | < 0.01 |
| Anemia (hemoglobin < 12 g%) | 23 (45.09%) | 81 (73.64%) | < 0.001 |
| Hematocrit % | 35.32 ± 7.58 | 31.34 ± 7.76 | < 0.01 |
| Hematocrit < 38% | 27 (52.94%) | 88 (80%) | < 0.001 |
| Red blood cells (million/μL) | 4.10 ± 0.93 | 3.59 ± 0.97 | < 0.01 |
| Red blood cells (< 4.5 million/μL) | 27 (52.94%) | 88 (80%) | < 0.001 |
| Total leukocyte count (cells/μL) | 7,500 (5,100–12,100) | 9,550 (6,800–12,300) | 0.12 |
| Leukocytosis (> 11,000 cells/μL) | 14 (27.45%) | 34 (30.91%) | 0.90 |
| Leucopenia (< 4,000 cells/μL) | 5 (9.8%) | 10 (9.09%) | |
| Absolute neutrophil count | 4,500 (2,838–7,854) | 5,590 (3,881–9,143) | < 0.05 |
| Absolute lymphocyte count | 2,268 (1,504–3,360) | 2,112 (1,310–2,913) | 0.16 |
| Absolute monocyte count | 384 (279–840) | 545 (310–912) | 0.11 |
| Absolute eosinophil count | 166 (72–264) | 122 (77–218) | 0.46 |
| Neutrophil to lymphocyte ratio | 2.13 (1.33–2.75) | 2.78 (1.79–4.75) | < 0.01 |
| Platelet count (×103 cells/μL) | 169.0 (122.0– 246.0) | 168.0 (105.8–217.3) | 0.24 |
| Thrombocytopenia (< 150 × 103 cells/μL) | 19 (37.25%) | 47 (42.73%) | 0.60 |
| Coagulation profile | |||
| Prothrombin time (s) | 15.40 (12.70–20.60) | 15.20 (12.80–17.60) | 0.77 |
| Prolonged prothrombin time | 6 (11.76%) | 8 (7.27%) | 0.34 |
| Activated partial thromboplastin Time (aPTT) (s) | 32.65 (27.78–40.10) | 30.10 (23.60–38.60) | 0.21 |
| Prolonged aPTT | 4 (7.84%) | 10 (9.09%) | 1.00 |
| International normalized ratio | 1.15 (0.93–1.57) | 1.12 (0.93–1.31) | 0.34 |
| Liver function test | |||
| Bilirubin total (mg/dL) | 0.80 (0.60–0.90) | 0.75 (0.50–1.20) | 0.99 |
| Hyperbilirubinemia | 10 (19.61%) | 37 (33.64%) | 0.06 |
| Total protein (gm/dL) | 6.62 ± 1.15 | 5.99 ± 0.95 | < 0.001 |
| Hypoproteinemia | 21 (41.18%) | 81 (73.64%) | < 0.0001 |
| Albumin (gm/dL) | 3.17 ± 0.85 | 2.51 ± 0.55 | < 0.0001 |
| Hypoalbuminemia | 40 (78.43%) | 106 (96.36%) | < 0.0001 |
| Globulin (gm/dL) | 3.44 ± 0.79 | 3.48 ± 0.76 | 0.77 |
| A/G ratio | 0.96 ± 0.33 | 0.75 ± 0.23 | < 0.0001 |
| Aspartate transaminase (IU/L) | 36 (26–75) | 48 (30.00–75.50) | 0.12 |
| High aspartate transaminase | 23 (45.10%) | 70 (63.64%) | < 0.05 |
| Alanine transaminase (IU/L) | 32 (21–57) | 34.00 (19.75–57.75) | 0.98 |
| High alanine transaminase | 17 (33.33%) | 44 (40%) | 0.41 |
| Alkaline phosphatase (IU/L) | 73 (62–108) | 81.50 (58.0–117.8) | 0.37 |
| High alkaline phosphatase | 8 (15.69%) | 25 (22.73%) | 0.30 |
| Renal function test | |||
| Urea (mg/dL) | 32.00 (22.00–47.00) | 36.00 (26.00–90.50) | 0.08 |
| Uremia | 11 (21.57%) | 43 (39.09%) | < 0.05 |
| Creatinine (mg/dL) | 0.80 (0.50–1.00) | 0.80 (0.60–1.85) | 0.14 |
| Hypercreatinemia | 10 (19.61%) | 32 (29.09%) | 0.20 |
| Uric acid (mg/dL) | 4.00 (3.50–5.30) | 4.90 (3.57–7.62) | 0.07 |
| Hypouricemia | 1 (1.96%) | 4 (3.64%) | 0.18 |
| Hyperuricemia | 7 (13.73%) | 28 (25.45%) | |
| Calcium (mg/dL) | 9.09 ± 0.44 | 7.67 ± 0.69 | < 0.0001 |
| Phosphate (mg/dL) | 3.70 (2.80–4.60) | 3.50 (2.57–5.25) | 0.96 |
| Hypophosphatemia | 11 (21.57%) | 24 (21.82%) | 0.81 |
| Hyperphosphatemia | 13 (25.49%) | 33 (30%) | |
| Electrolytes | |||
| Sodium (mEq/L) | 137.10 ± 5.63 | 134.90 ± 13.16 | 0.24 |
| Hyponatremia | 16 (31.37%) | 45 (40.91%) | 0.41 |
| Hypernatremia | 2 (3.92%) | 6 (5.45%) | |
| Potassium (mEq/L) | 4.31 ± 0.82 | 4.36 ± 0.89 | 0.71 |
| Hypokalemia | 6 (11.77%) | 14 (12.73%) | 0.86 |
| Hyperkalemia | 0 (0) | 0 (0) | |
Note: Continuous data are presented as mean ± standard deviation or median ± interquartile range. Categorical data are presented as frequency (%). Bold values signify statistical significance.
Significantly lower hemoglobin level was observed in the hypocalcemic patients as compared with the normocalcemic patients (p < 0.01). Also, a considerably lower hematocrit (p < 0.01) and red cell count (p < 0.01) were found in the hypocalcemic COVID-19 patients. Significantly higher (73.64%) numbers of patients were presented with anemia in the hypocalcemic group (p < 0.01). Absolute neutrophil count (ANC; < 0.05) and NLR (p < 0.01) were significantly higher in the hypocalcemic patients. Significantly lower levels of total protein and albumin were observed in the COVID-19 patients having hypocalcemia (p < 0.001 and p < 0.0001, respectively). In comparison with normocalcemic patients, significantly higher number of hypocalcemic patients had hypoproteinemia (73.64 vs. 41.18%, p < 0.0001) and hypoalbuminemia (96.36 vs. 78.43%, p < 0.0001). Albumin-to-globulin ratio was significantly decreased in the hypocalcemic patients as compared with the normocalcemic patients (p < 0.0001). Raised aspartate transaminase was observed in 63.64% of hypocalcemic patients compared with 45.10% of normocalcemic patients (p < 0.05). Altogether 39.09% of hypocalcemic patients had uremia compared with 21.57% of normocalcemic patients (p < 0.05).
Correlation of Albumin-Corrected Calcium with Different Laboratory Parameters
Albumin corrected calcium levels had significant positive correlation with hemoglobin levels (r = 0.20, p < 0.05), hematocrit (r = 0.22, p < 0.01), red cell count (r = 0.25, p < 0.01), total protein (r = 0.36, p < 0.0001), albumin (r = 0.46, p < 0.0001), and albumin-to-globulin ratio (r = 0.32, p < 0.0001). Significant negative correlation of the total calcium was observed with ANC (r = − 0.17, p < 0.05) and NLR (r = − 0.27, p < 0.001) (►Fig. 1).
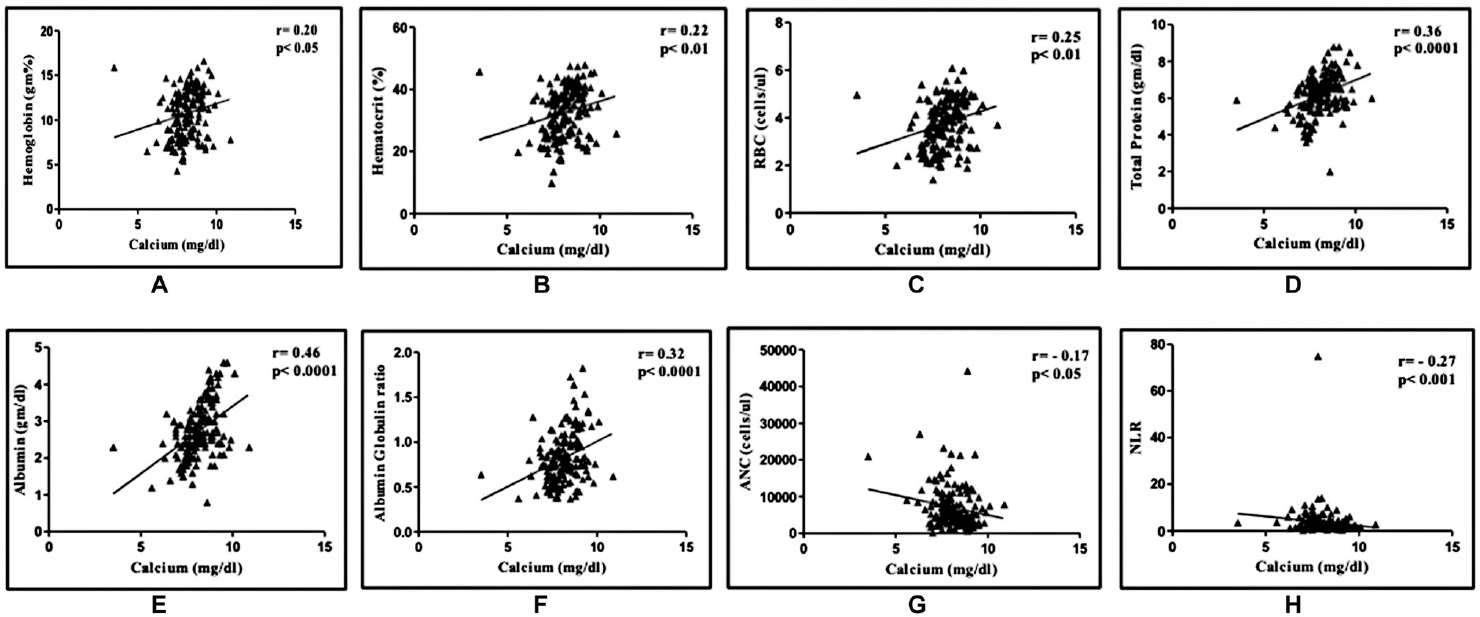
- Correlation of albumin corrected calcium with (A) hemoglobin, (B) hematocrit, (C) red blood cells (RBC), (D) total protein, (E)albumin, (F) albumin: globulin ratio, (G) absolute neutrophil count (ANC), (H) neutrophil: lymphocyte ratio (NLR).
Discussion
This study represents the first description of the hypocalcemic patients with COVID-19 disease from a COVID-19 care center in north India. The trends in the study show that the COVID-19 positive patients with hypocalcemia were severely affected by the disease and more patients required ventilator support during the hospital stay. A previous study demonstrates that hypocalcemia presents more frequently in male COVID-19 patients, similar to our findings.[12] Higher mortality was found in hypocalcemic patients; however, it was not found to be an independent predictor of mortality. The hypocalcemic group majorly comprised of more elderly subjects and ones with high-risk comorbidities and thus may have contributed to the disease severity and deaths.
In COVID-19 patients, hypocalcemia may be a result of an imbalance in parathyroid hormone and vitamin D levels in blood.[15] Also, the increased levels of unbound fatty acids and unsaturated fatty acids seen in severe COVID-19 patients may bind with the calcium and result into acute hypocalcemia.[16] In these patients, hypocalcemia positively correlates with reduced pulmonary functional index, lymphopenia, albumin levels, and vitamin D levels and negatively correlates with parathyroid hormone, C-reactive protein, lactate dehydrogenase, and D-dimer.[11,15,17] The current study also found a significant positive correlation between calcium and hemoglobin, hematocrit, red cell count, total protein level, albumin level, and albumin-to-globulin ratio and significant negative correlation of calcium with ANC and NLR.
The consequences in SARS-CoV-2 may be the same as SARS-CoV due to similarities in the genome of both the viruses.[18] Various viral pneumonia present with hypocalcemia.[19] Hypocalcemia has been reported in 60% of SARS diseases and 62% of Ebola virus diseases.[20] Altered calcium concentrations are usually observed during host cell dysfunction after viral infections.[21] Viruses use the calcium signal to generate a better environment for their benefits.[22] Changes in ion homeostasis, mainly in calcium homeostasis, promote the viral growth.[23] For various enveloped viruses such as SARS-CoV, MERS-CoV, and Ebolavirus, calcium plays a very important role in the viral fusion and promotes their replication by directly interacting with the fusion peptides of these viruses.[20] By using the calcium signal system of the host, virus can affect the occurrence and progression of the disease.[24] Calcium is required for the virus structure formation, viral entry, gene expression, virion maturation, and release. Calcium ion activity is demonstrated in small transmembrane protein coded by SARS-CoV E gene in animal models infected with SARS-CoV and its synthesis is increased during viral infection.[18] By using calcium channels or pump, viruses disturb the homeostasis of calcium in the body and induce host cell morbidity.[21] A high viral load and a prolonged period of viral shedding may present in COVID-19 hypocalcemic patients. Along with hypochloremia and bilateral pneumonia, hypocalcemia on admission has been shown to be an independent risk factor for long-term hospitalization in COVID-19 patients.[25]
Abnormal calcium level is commonly observed in critically ill patients. Studies show the association of hyper and hypocalcemia with higher organ injury and increased mortality in critical illnesses.[15,26,27] Up to 85% of the critically ill patients have presented with hypocalcemia and have higher mortality.[28] Very high prevalence of hypercalcemia, nearly up to 80%, has also been observed in the COVID-19 patients.[20] In our study, we found two-thirds of the COVID-19 patients to be hypocalcemic. Hypercalcemia has been included among the two most powerful risk factors to assess the severity of COVID-19 disease along with dyspnoea.[24]
Calcium levels should be monitored in all cases of acute and recovered COVID-19 patients.[29] Hospitalized patients with serum ionized calcium level below 4.80 mg/dL have an increased risk of acute respiratory failure and had more requirement of mechanical ventilation.[30] Timely supplementation of calcium has been suggested in severe COVID-19 patients to prevent organ failure as an early diagnosis and treatment of hypocalcemia may alleviate organ injury.[31]
Limitation and Future Needs
The study has a few limitations. It is a single-center study, and the sample size is relatively small, we believe that larger studies are needed to confirm our findings. Additionally, estimation of ionized serum calcium is more accurate than albumin-corrected calcium. And further studies are needed to find the causes of hypocalcemia in COVID-19 patients and to see whether the correction of hypocalcemia would lead to the improvement of outcomes.
Conclusion
The severity, ventilator requirement, and mortality were considerably higher in the hypocalcemic COVID-19 patients. The current study indicates age, comorbidities, low hemoglobin level, higher NLR, and hypoalbuminemia are associated with poor outcomes.
Authors' Contributions
B.S.P. wrote the manuscript; B.S.P., T.M., and A.S. did data analysis; B.S.P., T.M., R.A., K.S., N.N., D.S., and Surbhi helped in data collection, clinical correlation, and interpretation; R.M.P. helped in statistical studies and interpretation; N.W., R.M., and A.T. helped in editing/review of the manuscript. A.S. is guarantee for the study.
Ethical Approval
The study was conducted after obtaining the approval from the Institutional Ethics Committee.
Conflict of Interest
None declared.
Funding
None.
References
- COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162 108142
- [CrossRef] [PubMed] [Google Scholar]
- Accessed November 03, 2020 at: https://www.who.int/
- Accessed November 03, 2020 at: https://www.mohfw.gov.in/
- COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14(04):513-517.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of 2019 coronavirus pneumonia (COVID-19): an updated systematic review. MedRxiv 2020 DOI: 10.1101/2020.03.07.20032573
- [CrossRef] [Google Scholar]
- China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720.
- [CrossRef] [Google Scholar]
- Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372-2374.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and risk factors for disease severity and death in patients with coronavirus disease 2019 in Wuhan, China. Front Med (Lausanne). 2020;7:532.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(02):e16-e25.
- [CrossRef] [PubMed] [Google Scholar]
- C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14 1753466620937175
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of hypocalcemia in COVID-19. Clin Res Diabetes Endocrinol. 2020;3(01):1-3.
- [CrossRef] [Google Scholar]
- Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68(03):475-478.
- [CrossRef] [PubMed] [Google Scholar]
- Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018;517:3-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ca2+ ions promote fusion of middle east respiratory syndrome coronavirus with host cells and increase infectivity. J Virol. 2020;94(13):e00426-e20.
- [CrossRef] [PubMed] [Google Scholar]
- Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY). 2020;12(12):11287-11295.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanistic basis and therapeutic relevance of hypocalcemia during severe COVID-19 infection. Endocrine. 2020;70(03):461-462.
- [CrossRef] [PubMed] [Google Scholar]
- Low serum calcium and phosphorus and their clinical performance in detecting COVID-19 patients. J Med Virol. 2021;93(03):1639-1651.
- [CrossRef] [PubMed] [Google Scholar]
- Low levels of total and ionized calcium in blood of COVID-19 patients. Clin Chem Lab Med. 2020;58(09):e171-e173.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory abnormalities in patients with bacterial pneumonia. Chest. 1997;111(03):595-600.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord. 2020;21(04):495-507.
- [CrossRef] [PubMed] [Google Scholar]
- Host calcium channels and pumps in viral infections. Cells. 2019;9(01):94.
- [CrossRef] [PubMed] [Google Scholar]
- Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46(01):1-17.
- [CrossRef] [PubMed] [Google Scholar]
- Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330-339.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction model of severe coronavirus disease 2019 (COVID-19) cases shows the leading risk factor of hypocalcemia. Research Square 2020; DOI: 10.21203/rs.3.rs-41318/v1
- [Google Scholar]
- Risk factors associated with long-term hospitalization in patients with COVID-19: a single-centered, retrospective study. Front Med (Lausanne). 2020;7:315.
- [CrossRef] [PubMed] [Google Scholar]
- Association of serum total and ionized calcium with all-cause mortality in critically ill patients with acute kidney injury. Clin Chim Acta. 2019;494:94-99.
- [CrossRef] [PubMed] [Google Scholar]
- Association of initial serum total calcium concentration with mortality in critical illness. BioMed Res Int. 2018;2018 7648506
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of pulmonary arterial pressure using critical care echocardiography: dealing with the yin and the yang? Crit Care Med. 2019;47(01):126.
- [CrossRef] [Google Scholar]
- Endocrine vigilance in COVID-19. J Pak Med Assoc. 2020;70(05):S83-S86.
- [CrossRef] [PubMed] [Google Scholar]
- Serum ionised calcium and the risk of acute respiratory failure in hospitalised patients: a single-centre cohort study in the USA. BMJ Open. 2020;10(03):e034325.
- [CrossRef] [PubMed] [Google Scholar]
- Hypocalcemia and hypoalbuminemia during COVID-19 infection: opportunities for therapeutic intervention. J Infect Public Health. 2020;13(12):1887.
- [CrossRef] [PubMed] [Google Scholar]






