Translate this page into:
Changing Trends of Vulvovaginal Candidiasis
Address for correspondence: Dr. Tumkur Anjaneya Dhanalakshmi, E-mail: dhans07_adi@yahoo.co.in
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Vulvovaginal candidiasis is one of the most common infections seen in women.
Materials and Methods:
A total of 300 symptomatic women were studied. High vaginal swabs collected from each patient were processed by Gram stain, culture on Sabourauds dextrose agar and CHROM agar plates. Isolates were identified and speciated using conventional methods and by the color of the colonies on the CHROM agar. Antifungal susceptibility was performed by disc diffusion method for fluconazole (25 μg) and voriconazole (1 μg) discs as per Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results:
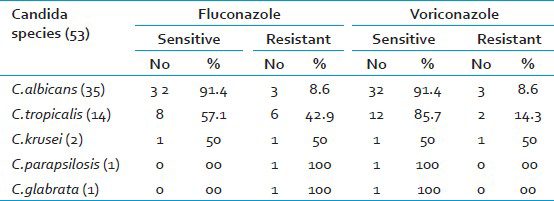
Vulvovaginal candidiasis was found in 53 (17.7%) of cases. Gram stain was positive in 22 (41.41%) of culture positives. Speciation of isolates by conventional and CHROM agar methods showed similar results. C. albicans 35 (66.0%) was the most common species isolated followed by C. tropicalis 14 (26.4%), C. krusei 2 (3.8%), C. parapsilosis and C. glabrata in 1 (1.9%) case each. Sensitivity to fluconazole was found in 91.4% of C. albicans, 57.1% of C. tropicalis and 50.0% of C. krusei. Sensitivity to voriconazole was seen in 91.4% of C. albicans, 85.7% of C. tropicalis and 50.0% of C. krusei. C. parapsilosis and C. glabrata were found sensitive only to voriconazole.
Conclusion:
CHROM agar has the advantage of being rapid, simple and cost effective method as compared to conventional methods in speciation of Candida. Routine susceptibility testing of Candida isolates help in selecting the most appropriate antifungal agent for vulvovaginal candidiasis.
Keywords
CHROM agar
fluconazole
voriconazole
vulvovaginal candidiasis
INTRODUCTION
Candidiasis is the most common vaginal infection in most countries affecting about 50-72% of women, 40-50% having recurrent episodes.[12] Vaginal candidiasis if untreated can lead to chorioamnitis with subsequent abortion and prematurity in pregnant women, congenital infection of the neonate and pelvic inflammatory disease resulting in infertility in non-pregnant women.[34] The lack of specificity of symptoms and signs in vulvovaginal candidiasis explains the need for laboratory confirmation by culture.[5] Candida albicans is the most commonly isolated species. More recently non albicans Candida (NAC) species have been recovered with increasing frequency,[4] which are known for their variable resistance to azoles.[67] To avoid selection of less susceptible NAC species by empirical anti-fungal treatment or prophylaxis, speciation of Candida isolates is essential in routine specimen processing.[7] Present study was undertaken to determine the prevalence of vaginal candidiasis in symptomatic pregnant and non-pregnant women and to evaluate the advantages of CHROM agar over conventional methods in speciation of Candida. Antifungal susceptibility testing was done by disc diffusion method using fluconazole and voriconazole discs.
MATERIALS AND METHODS
A total of 300 women in the age group 19-50 years with clinically suspected vulvovaginal candidiasis (VVC) who were referred from the Department of Obstetrics and Gynecology from November 2011-October 2012 formed the study group. Ethical clearance was obtained from the institution. Two high vaginal swabs were collected from each patient and processed in the Department of Microbiology. One swab was used for Gram staining and the other swab to inoculate Sabourauds dextrose agar (SDA) tubes with chloramphenicol and CHROM agar plates (Hi Media Mumbai, India). The inoculated tubes and plates were incubated at 37°C and 35°C, respectively for 48 hours. Isolates on SDA tubes were identified and speciated using conventional methods i.e. germ tube test, sugar assimilation test and morphology on corn meal agar as per standard methods.[89] The isolates on CHROM agar were identified by noting the color of the colonies.[Figure 1] Antifungal susceptibility testing was performed by disc diffusion method for fluconazole 25 μg and voriconazole 1 μg discs (Hi Media Mumbai, India) using control strains as per CLSI guidelines.[10]
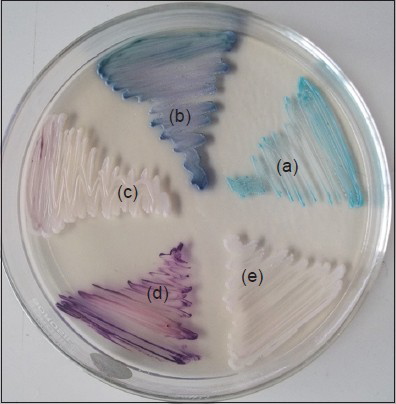
- CHROM agar showing different species of Candida (a) C.albicans (b) C.tropicalis (c) C.krusei (d) C.glabrata (e) C.parapsilosis
RESULTS
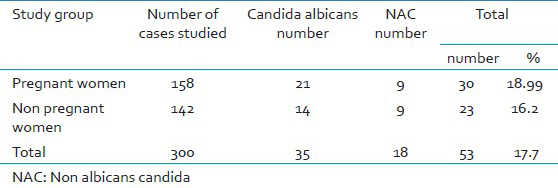
Of the 300 symptomatic women studied, 158 (52.7%) were pregnant women and 142 (47.3%) were non-pregnant women. Vaginal candidiasis was found in 53 (17.7%) women by culture. Gram stain was positive in 22 (41.41%) of culture positives. Speciation by conventional and CHROM agar method showed C.albicans in 35 (66%) cases, C.tropicalis in 14 (26.4%), C.krusei in 2 (3.8%), C.parapsilosis and C.glabrata in 1 (1.9%) case each. Table 1 shows the C.albicans and NAC isolated among the study group. Table 2 shows the antifungal susceptibility pattern of the isolates to fluconazole and voriconazole.
DISCUSSION
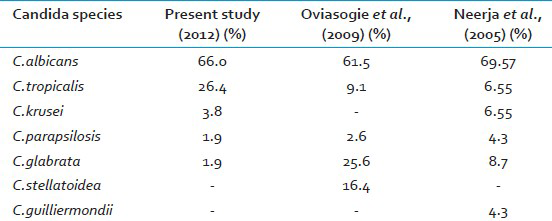
Bacterial vaginosis, Candidiasis, and Trichomoniasis are responsible for 90% of cases of vaginal infection.[1] In the present study, vaginal candidiasis was found in 53 (17.7%) of symptomatic women which is lower than other studies.[45] This difference in prevalence may be attributed to the study group.[7] In the present study 30 (10%) pregnant women 23 (7.7%) non- pregnant women had VVC with a narrow differential margin. The increased incidence of vaginal candidiasis in pregnant women may be due to elevated levels of progesterone and estrogen. Progesterone has suppressive effects on the anti-Candida activity of neutrophils. Estrogen has been found to reduce the ability of vaginal epithelial cells to inhibit the growth of Candida.[3] The possible reason for the narrow differential margin in the prevalence of VVC noted in pregnant and non-pregnant women may be because of change in women's health quality, resistance of the Candida species to azoles, failure to eradicate Candida from female genital tract,[1] and due to increased use of oral contraceptive pills (OCPs). Many investigators continue to identify OCP as a predisposing factor. This might be because of similarity between the mechanism operating in pregnancy and high estrogen OCP in increasing vaginal colonization of Candida.[5] Vaginal culture was the most sensitive method as compared to Gram stain as noted in other study.[3] In the present study, similar results were obtained regarding speciation of Candida by conventional and CHROM agar methods but, CHROM agar has the advantage of being rapid, simple and cost effective as compared to conventional methods which are slow, technically demanding and expensive.[611] In the present study, C.albicans was the most common species isolated 35 (66.0%) followed by NAC 18 (34%) as in other studies.[45] C.albicans adheres to vaginal epithelial cells in significantly higher numbers than do other Candida species. This could explain the relative infrequency of the latter in vaginal candidiasis.[3] Table 3 shows the Candida species isolated in various studies. Current trend shows an increase in the prevalence of NAC vaginitis and is significantly higher in the present study.[23] The possible reason for this may be the increased indiscriminate usage of antimycotics which eliminates the more sensitive C.albicans and selects more azole-resistant NAC species.[2] In the present study, C.tropicalis (14, 78%) was the most common NAC species isolated unlike in other studies where C.glabrata, C.stellatoidea or C.krusei was the major isolate.[345] Earlier it was stated that, given the rarity of VVC caused by resistant C.albicans strains, susceptibility testing was rarely indicated[2] but in the present study 9% of C.albicans was found resistant to both fluconazole and voriconazole. The resistance of C.albicans to azoles has also been reported by others.[912] Hence there is a need for routine antifungal susceptibility testing of all Candida isolates. In the present study, C.albicans and majority of NAC species were found susceptible to voriconazole as compared to fluconazole [Table 2]. Voriconazole can be preferred over fluconazole as it has broad spectrum activity against Candida species which are inherently resistant to fluconazole.[9]
CONCLUSION
Direct inoculation of the specimen to CHROM agar will help early speciation and detection of mixed infections.[6] Changing trends in the antifungal susceptibility towards fluconazole recommends routine antifungal susceptibility testing of Candida isolates in clinical microbiology laboratories. Presumptive identification followed by confirmation of yeast species helps to initiate early appropriate antifungal therapy thereby reducing the morbidity and mortality.[7]
ACKNOWLEDGMENT
We are thankful to all our colleagues and technicians for their support.
Source of Support: Nil
Conflict of Interest: There is no conflict within authors for submitting and publishing of this research article.
REFERENCES
- Frequency and etiology of vulvovaginal candidiasis in women referred to a gynecological center in Babol, Iran. Int J Fertil Steril. 2009;3:74-7.
- [Google Scholar]
- Gram stain versus culture in the diagnosis of vulvovaginal Candidiasis. East Mediterr Health J. 2001;7:925-34.
- [Google Scholar]
- Candida species amongst pregnant women in Benin city, Nigeria: Effect of predisposing factors. Afr J Clin Exper Microbiol. 2009;10:92-8.
- [Google Scholar]
- Significance of Candida culture in women with vulvovaginal symptoms. J Obstet Gynecol India. 2006;56:139-41.
- [Google Scholar]
- Yeast identification in routine clinical microbiology laboratory and its clinical relevance. Indian J Med Microbiol. 2011;29:172-7.
- [Google Scholar]
- Fungi. In: Collee JG, Frazer AG, Marmion BP, Simmons A, Mackie McCartney, eds. Practical Medical Microbiology (14th ed). Edinburgh: Churchill Living Stone; 1996. p. :697-717.
- [Google Scholar]
- Text book of Medical Mycology. (2nd ed). New Delhi: Mehtha Publishers; 2002. p. :212-27.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts; Approved guidelines. (2nd ed). Wayne: CLSI; 2009. p. :29. M-44-A2
- [Google Scholar]
- Hichrom candida agar for identification of Candida species. Indian J Pathol Microbiol. 2010;53:93-5.
- [Google Scholar]
- Determination of antifungal susceptibility patterns among the clinical isolates of Candida species. J Glob Infect Dis. 2011;3:357-60.
- [Google Scholar]





