Translate this page into:
Clinical and microbiology profile of typhoidal and nontyphoidal Salmonella blood stream infection: An observational study from a university hospital
*Corresponding author: Chinmoy Sahu, MD, PDCC, Department of Microbiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow 226014, Uttar Pradesh, India sahu.chinmoy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Siddiqui T, Sinha R, Patel SS, Sahu C. Clinical and microbiology profile of typhoidal and nontyphoidal salmonella blood stream infection: an observational study from a university hospital. J Lab Physicians. 2024;16:188-93. doi: 10.1055/s-0043-1772216
Abstract
Objectives:
Species distribution and antibiotic resistance pattern of Salmonella varies with time and place. Rapid and correct use of antibiotics requires understanding of the distribution and drug resistance patterns. Therefore, we aimed to study the clinical profile of patients along with speciation and antibiogram of Salmonella isolates from blood.
Materials and Methods:
It is an observational study, conducted from December 2019 to December 2021 at our university hospital. Blood cultures were processed by automated blood culture system. Salmonella isolates were identified by their morphological properties, biochemical reaction, and serological tests. Antibiotic susceptibility pattern was assessed by Kirby–Bauer’s disc diffusion method and Phoenix automated system.
Results:
The male-to-female ratio of the patients in the study was 2.5:1 and the mean age of the patients was 11.7 years (1–27 years). Salmonella spp. was isolated from 21 patients out of 1,350 positive blood cultures. Nine isolates were identified as S. Typhi seven as S. Choleraesuis three as S. Paratyphi A and two as S. Paratyphi B. Immuno-compromised conditions were significantly associated in nontyphoidal Salmonella bacteremia (p = 0.0253). Isolates showed maximum resistance toward nalidixic acid (100%) followed by fluoroquinolones (52.4%). Multidrug resistance, extreme drug resistance, and azithromycin resistance was seen in 14.3, 4.8, and 4.8% isolates, respectively. Posttreatment recovery was observed in 20 patients.
Conclusions:
Emergence of S. Choleraesuis among Salmonella spp. in blood stream infection, next only to S. Typhi was noted. Rising drug resistance is a matter of concern.
Keywords
Salmonella
nontyphoidal Salmonella
S. Choleraesuis
Salmonella antibiotic resistance
INTRODUCTION
Invasive Salmonella infection has emerged as a major public health concern especially in low resource regions.[1] Poor public health, lack of adequate sanitation, shortage of clean water, and low socioeconomic condition make it endemic in developing countries.[2,3] Salmonella is the most complex genus of the Enterobacteriaceae family and can be commonly divided into typhoidal (TS) and nontyphoidal Salmonella (NTS) based on their phenotypes. The predominant organism in South East Asia is Salmonella Typhi[1,3] while NTS predominates in Africa.[1,4-8] Clinical manifestation and severity of infection ranges from self-limiting infections to life-threatening sepsis. NTS infection usually produces gastrointestinal symptoms which are usually self-limiting and lasts for 3 to 7 days; however, a small group of patients (immunocompromised individuals, infants, elderly, and patients with underlying disease) may develop invasive blood stream infections (BSIs) necessitating antimicrobial treatment.
As the burden of antimicrobial resistance in Salmonella is rising, there is a surge in the multidrug resistance (MDR) and fluoroquinolone resistance infections which lead to adverse clinical outcomes.[9,10] Changes in the susceptibility pattern for drugs like ceftriaxone and azithromycin is the major concern. Species distribution and antibiotic resistance pattern of Salmonella varies with time and place. Studies from our country have shown that MDR in Salmonella varies from approximately 2 to 4%.[11,12] Better knowledge of the spectrum and antibiogram of the infective agent of a particular geographical region will aid in the diagnosis and management of patients and will also help in the formulation of hospital antibiotic policy. The present study was undertaken to analyze the clinical profile, treatment, and outcome of patients suffering from Salmonella bacteremia and to examine the species and antibiogram of blood isolates at a tertiary care hospital in North India.
MATERIALS AND METHODS
Study Setting and Duration
This is a prospective observational study done from December 2019 till December 2021 in a microbiology laboratory of a 1,600-bedded tertiary care center of Northern India.
Inclusion Criteria
All consecutive, non repeat Salmonella isolates obtained from blood cultures received in the microbiology laboratory during the study period were included in the study.
Identification and Antibiotic Susceptibility Testing
All blood cultures were performed using BACTEC 9120 vials (Becton, Dickinson and Company, Franklin Lakes, USA) and incubated in the instrument for five consecutive days as per the standard guidelines. After the bottles flagged positive, Gram-staining with subcultures on appropriate culture media was done. Phenotypic identification (ID) of bacterial isolate was performed by studying its colony morphology and standard biochemical tests. Kaufmann– White scheme was used for typing of isolates using polyvalent O antiserum and serotype-specific antisera (Salmonella agglutinating serum, Bio-Rad Laboratories India Pvt. Ltd., Haryana, India). The isolates identified as Salmonella were also subjected to ID and antimicrobial susceptibility testing using Phoenix NMIC/ID-55 panels (Becton, Dickinson and Company) for drugs like ampicillin, chloramphenicol, sulfamethoxazole trimethoprim, and ciprofloxacin. Kirby– Bauer’s disc diffusion method was performed for other drugs (nalidixic acid [30 μg], ampicillin [10 μg], ceftriaxone [30 μg], chloramphenicol [30 μg], azithromycin [15 μg], ciprofloxacin [5 μg], and cefixime [5 μg]). All antibiotic discs were procured from Oxoid India Ltd. Results were interpreted using the Clinical and Laboratory Standards Institute (CLSI 2020) breakpoints.[13] A standard strain of Escherichia coli ATCC 25922 was included as quality control. Isolates were considered MDR if they were resistant to ampicillin/amoxicillin, chloramphenicol, and trimethoprimsulfamethoxazole and extensively drug resistant (XDR) if the isolates were MDR along with resistance toward fluoroquinolone and any third-generation cephalosporins. Isolates were considered fluoroquinolone non susceptible if they demonstrated intermediate susceptibility or were non susceptible to ciprofloxacin.[14]
Data Collection and Patient Follow-Up
Medical records for all the patients showing blood culture positive for Salmonella spp. was analyzed for details like age, gender, comorbid conditions, risk factors, laboratory parameters, and antibiotics administered. Details were recorded in a predesigned pro forma. Follow-up visits of the patient after discharge were recorded from the outpatient department for the treatment outcomes.
RESULTS
Over a period of 2 years (December 2019–December 2021), a total of 28,257 blood samples from patients of clinically suspected septicemia were microbiologically evaluated. Bacterial growth was observed in 1,350 (4.78%) of the cultured samples. Salmonella was isolated from 21 patients. The male to-female ratio of the patients in the study was 2.5:1 and the mean age of the patients was 11.7 years (range 1–27 years). No gender or age group was significantly associated with TS and NTS in the study; however, 71.5% (10/14) of TS was isolated from children (0–10 years) and 85.7% (6/7) NTS was isolated mainly from BSI of young adults (15–30 years) in the study (Table 1).
| Clinico-demographic parameters | Typhoidal Salmonella (n, %) |
Nontyphoidal Salmonella (n, %) |
p-Value |
|---|---|---|---|
| Gender | |||
| Male | 11 (78.6) | 4 (57.1) | 0.306 |
| Female | 3 (21.4) | 3 (42.9) | |
| Age group (y) | |||
| 0–5 | 7 (50) | 0 (0) | 0.109 |
| 05–10 | 3 (21.4) | 1 (14.3) | |
| 10–15 | 2 (14.3) | 1 (14.3) | |
| 15–20 | 1 (7.1) | 3 (42.8) | |
| 20–25 | 1 (7.1) | 1 (14.3) | |
| 25–30 | 0 (0) | 1 (14.3) | |
| Symptoms | |||
| Fever | 13 (92.9) | 6 (85.7) | 0.186 |
| Diarrhea | 2 (14.3) | 2 (28.6) | |
| Comorbidities/Risk factors | |||
| Hematological malignancy | 2 (14.3) | 2 (28.6) | 0.677 |
| Solid organ malignancy | 1 (7.1) | 0 (0) | |
| Metabolic disorders | 1 (7.1) | 1 (14.3) | |
| Rheumatological disorders | 0 (0) | 2 (28.6) | |
| Chronic liver disease | 1 (7.1) | 1 (14.3) | |
| Chronic heart disease | 1 (7.1) | 0 (0) | |
| Immunosuppressive drug use | 0 (0) | 2 (28.6) | |
| Infant of diabetic mother | 1 (7.1) | 0 (0) | |
| Postcholecystectomy | 0 (0) | 1 (14.3) | |
| Immunosuppression | |||
| Present | 7 (50) | 7 (100) | 0.0253 |
| Absent | 7 (50) | 0 (0) | |
| Outcome | |||
| Recovered | 13 (92.9) | 7 (100) | 0.469 |
| Died | 1 (7.1) | 0 (0) | |
| Laboratory investigation | |||
| Total leucocyte count (TLC), median (IQR),/μL [median (Q1, Q3)] | 8,500 (3,100, 17,400) | 8,100 (5,600, 18,900) | 0.056 |
| Median time for culture positivity (h) [median (Q1, Q3)] | 11.2 (9.2, 13.2) | 16 (13, 17) | 0.689 |
Abbreviation: IQR, interquartile range.
Of the 21 isolates of Salmonella, Salmonella enterica subspecies serovar Typhi was the most prevalent bacterial isolate (9, 43%), followed by Salmonella enterica serovar Choleraesuis (7, 33.3%) followed by S.Paratyphi A (3, 14%) and S. Paratyphi B (2, 10%) (Figure 1). Among NTS only S. Choleraesuis was isolated in the present study. Immunocompromised states like hematological malignancy, solid organ tumors, autoimmune disorders, or use of anticancer drugs, steroids, or other immunosuppressive agents were present in all NTS and 50% patients of TS (p = 0.0253), but no single comorbid condition was significantly associated with NTS. Fever alone was the most common presenting symptom (19/21) followed by diarrhea (4/21) in both TS and NTS. Total leucocyte count varied from 3,100 to 17,400/μL (mean count = 8,500/μL) in TS patients and 5,600 to 18,900/μL (mean count = 8,100/μL) in NTS patients, respectively. The median time to positivity of blood culture for TS and NTS was 11.2 and 16 hours, respectively, indicating NTS takes more time for growth in blood culture and the turn-around time for culture reports, including antimicrobial sensitivity results, was between 48 and 72 hours.
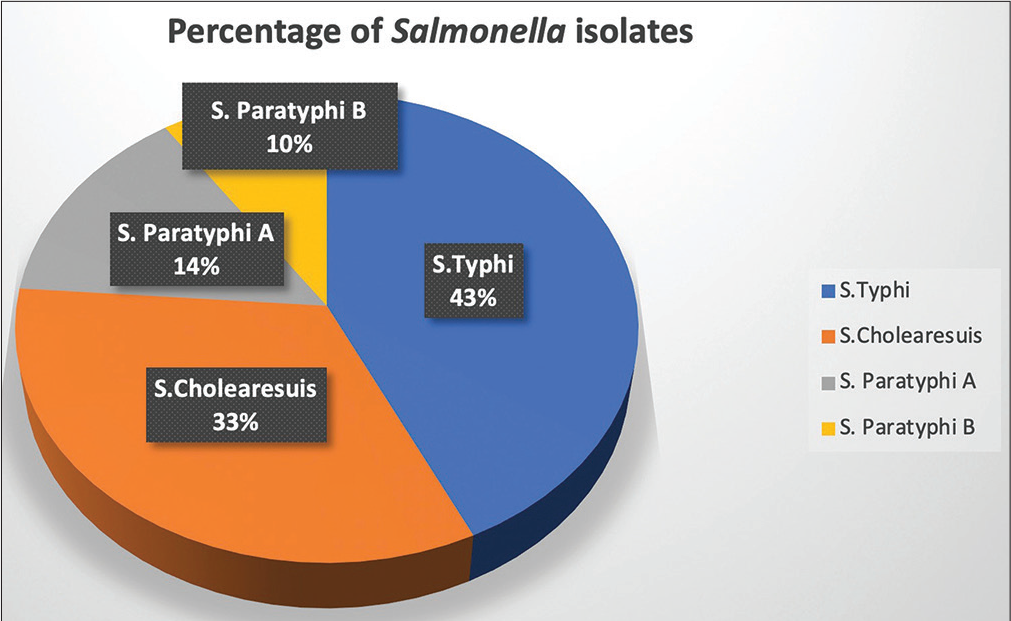
- Distribution of Salmonella isolates of the study.
Details of antimicrobial susceptibility of Salmonella isolates are shown in Table 2. Highest resistance was observed for nalidixic acid (100%, 21/21), followed by ciprofloxacin (76.2%, 16/21) and ampicillin (28.6%, 6/21). MDR was seen in only 14.3% (3/21) of the Salmonella enterica isolates (two S. Typhi and one S Paratyphi B) (Figure 2). Resistance to azithromycin was observed in one XDR isolate which was identified as S. Paratyphi B and this isolate was resistant to all antibiotics except carbapenems.
| Antibiotics tested | Typhoidal Salmonella (n = 14) | Nontyphoidal Salmonella (n = 7) | ||
|---|---|---|---|---|
| Susceptible | Resistant | Susceptible | Resistant | |
| Ampicillin | 9 (64.3%) | 5 (35.7%) | 6 (85.7%) | 1 (14.3%) |
| Chloramphenicol | 11 (78.6%) | 3 (21.4%) | 7 (100.00%) | 0 |
| Cotrimoxazole | 11 (78.6%) | 3 (21.4%) | 7 (100.00%) | 0 |
| Nalidixic acid | 0 | 14 (100%) | 0 (0.00%) | 7 (100%) |
| Ciprofloxacin | 3 (21.4%) | 11 (78.6%) | 2 (28.5%) | 5 (71.5%) |
| Ceftriaxone | 13 (92.8%) | 1 (7.2%) | 7 (100.00%) | 0 |
| Ceflxime | 13 (92.8%) | 1 (7.2%) | 7 (100.00%) | 0 |
| Azithromycin | 13 (92.8%) | 1 (7.2%) | 7 (100.00%) | 0 |
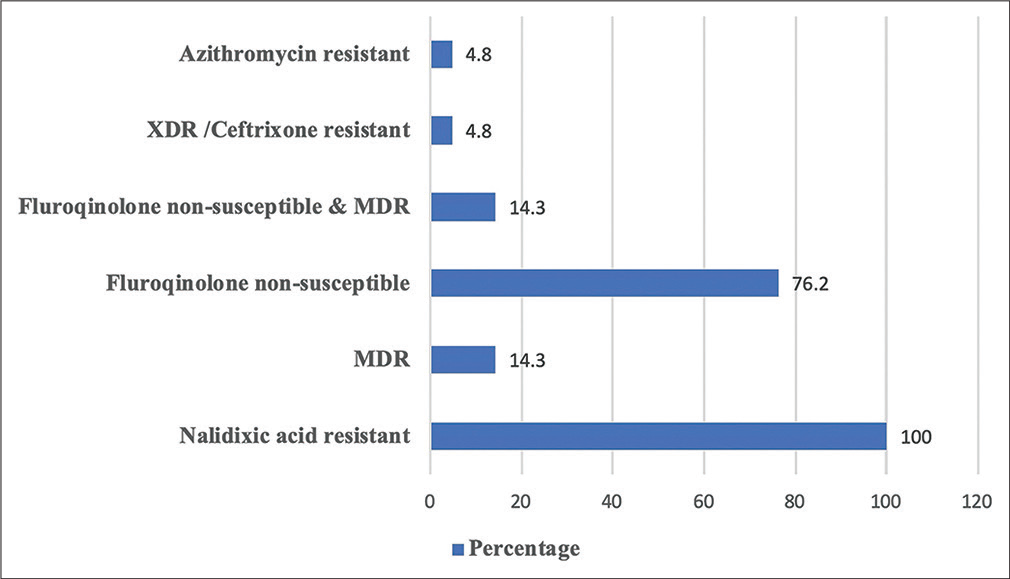
- Antibiotic resistant pattern of Salmonella isolates of the study. Abbreviations: MDR = Multidrug resistant (resistant to chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole); XDR = Extensively drug-resistant (fluoroquinolone non-susceptible, MDR, and resistant to third-generation cephalosporins eg. Ceftriaxone).
Patients received antibiotic treatment based on clinician’s decision and microbiology laboratory report stating the antibiogram of the organism isolated. Ensuing medication, 95.23% (20/21) patients recovered and one succumbed.
DISCUSSION
Although globally, Salmonella is considered familiar cause of gastrointestinal illness, it is also responsible for a significant number of invasive infections (BSI). Several studies have discussed the association of various pathogens in BSI and the overall morbidity and mortality.[15] Among the spectrum of pathogens reported from low-resource settings, burden of Salmonella is considerable.[16-18] In the current study, we were able to identify Salmonella in 1.56% (21/1350) of positive blood cultures. A similar hospital-based study from Delhi which investigated bacterial infection also exhibited a low (3.01%) prevalence.[19] An African study reported a high 28% (n = 67) positivity in pediatric patients among 242 culture positives which could be attributable to the selection of patients and the study was conducted in more than one hospital.[20] TS was mainly isolated from blood samples of children (0–10 years) which is in concordance with earlier studies.[3,6,20]
Small fraction of blood culture positive isolates account for NTS bacteremia. They are considered important among immunocompromised patients especially those suffering from malignancy and immune-deficiency syndrome.[21] The global burden of invasive disease caused by NTS is substantial as stated by Ao et al in their report.[22] Moreover, incidence in some regions are higher than expected. A study from Australia demonstrated an annual incidence of 6.5% invasive NTS (iNTS) disease.[23] Little is known about the incidence of iNTS in Asia.[24]
It is noteworthy that we were able to isolate and identify 7 (33.33%) NTS in our study. These were more frequently associated with BSI of young adults (15–30 years) which is in contrast to an African study where infants and young children were mainly affected.[25] Culture-confirmed S. Choleraesuis is the only serovar which was responsible for all the iNTS cases of our study which may be attributable to its highly invasive property as S. Choleraesuis is usually associated with bacteremia and extraintestinal focal infections in both children and adults, with little or no involvement of the gastrointestinal tract.[26-28] This could also be a possible explanation of the absence of diarrheal history in most cases of iNTS in our study.
The global epidemiology of NTS serotypes causing human BSIs varies greatly in different countries. In subSaharan African countries, S. Typhimurium followed by S. Enteritidis are the two most common serovars associated with bacteremia.[29] In India, only a few studies and reports have documented the isolation of NTS from blood, with no predominant serotype.[30,31] S. Choleraesuis has been reported from Thailand[27,32] and is the second common human Salmonellosis causing serotype in Taiwan.[26,27] The first case of bacteremia from India was from an adult febrile patient in whom S. Choleraesuis was isolated from both blood and urine cultures.[31] Recovery rate was good in our study and the death recorded in a patient infected with S. Typhi. Few of the previous studies have discussed high rates of mortality (47%) with NTS bacteremia which could be ascribed to the severity of the underlying disease and the serovars of NTS.[33]
Nalidixic acid resistance (surrogate marker for fluoroquinolone susceptibility) was present in all our isolates and majority was resistant to ciprofloxacin, ampicillin, chloramphenicol, and cotrimoxazole, which indicates misuse of these drugs to treat febrile illness of assumed bacterial origin. The isolates were susceptible toward third-generation cephalosporins and azithromycin. S. Paratyphi B showed considerable resistance toward the antibiotics which may be related to extensive use of these drugs leading to the development of resistance.[34] Rate of resistance among S. Choleraesuis isolates was low, though the isolates exhibited resistance toward ciprofloxacin and ampicillin. MDR was observed in three isolates which is very less compared with previous studies done in India.[34,35] Salmonella spp. continues to be an important cause of BSI and poses a major health problem. Recently, NTS due to S. Choleraesuis has been identified in immunocompromised individuals suffering from BSIs in Indian subcontinent,[30,31] so in future more antiseras against it should be kept available in microbiology laboratory. Also, increased occurrence of antimicrobial resistance is a global concern and developing countries are more prone to the catastrophic consequence. Therefore, upgradation in the diagnostic and surveillance system is needed to identify, characterize, capture the true burden and control the BSI, and antimicrobial resistance of Salmonella and NTS, for the better understanding of the epidemiology as it has implication in patient management and formulation of health policy.
Authors contribution
The authors declare that the manuscript has been read and approved by all the authors, the requirements for authorship have been met, and that each author believes that the manuscript represents honest work, study is original, and all authors agree on its findings.
Ethical approval
Ethical approval was obtained from Institutional Ethics Committee (Reference number: PGI/BE/274/2020).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Patients’ Consent
Written informed consent for publication of clinical details was obtained from the patients.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The burden of enteric fever. J Infect Dev Ctries. 2008;2:253-259.
- [CrossRef] [PubMed] [Google Scholar]
- Typhoid epidemiology, diagnostics and the human challenge model. Curr Opin Gastroenterol. 2014;30:7-17.
- [CrossRef] [PubMed] [Google Scholar]
- High mortality of infant bacteraemia clinically indistinguishable from severe malaria. QJM. 2004;97:591-597.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic bacteraemia in children presenting with clinical pneumonia and the impact of non-typhoid Salmonella (NTS) BMC Infect Dis. 2010;10:319.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemic increase in Salmonella bloodstream infection in children, Bwamanda, the Democratic Republic of Congo. Eur J Clin Microbiol Infect Dis. 2014;33:79-87.
- [CrossRef] [PubMed] [Google Scholar]
- Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis. 2009;49:606-611.
- [CrossRef] [PubMed] [Google Scholar]
- Invasive Salmonella infections in areas of high and low malaria transmission intensity in Tanzania. Clin Infect Dis. 2014;58:638-647.
- [CrossRef] [PubMed] [Google Scholar]
- Drugresistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis. BMC Med. 2020;18:1.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrugresistant pathogens. Clin Microbiol Infect. 2020;26:151-157.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and trends in the antimicrobial susceptibility pattern of Salmonella enterica serovars Typhi and Paratyphi A among children in a pediatric tertiary care hospital in South India over a period of ten years: a retrospective study. Eur J Clin Microbiol Infect Dis. 2017;36:2399-2404.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of typhoid and paratyphoid in India: systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004616.
- [CrossRef] [PubMed] [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing (30th ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
- [Google Scholar]
- Multidrug-resistant typhoid fever in children: epidemiology and therapeutic approach. Pediatr Infect Dis J. 1994;13:134-140.
- [CrossRef] [PubMed] [Google Scholar]
- Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2:10401.
- [CrossRef] [PubMed] [Google Scholar]
- Global typhoid fever incidence: a systematic review and meta-analysis. Clin Infect Dis. 2019;68:S105-S116.
- [CrossRef] [PubMed] [Google Scholar]
- Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis. 2012;12:480-487.
- [CrossRef] [PubMed] [Google Scholar]
- Characterisation of antimicrobial resistance in Salmonellae during 2014-2015 from four centres across India: an ICMR antimicrobial resistance surveillance network report. Indian J Med Microbiol. 2017;35:61-68.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and microbiological profile of blood culture positive Salmonella sp in Delhi: a hospital based study. Int J Sci Res. 2018;7:11-13.
- [Google Scholar]
- Salmonella bloodstream infections in hospitalized children with acute febrile illness-Uganda, 2016-2019. Am J Trop Med Hyg. 2021;105:37-46.
- [CrossRef] [PubMed] [Google Scholar]
- Non-typhoidal Salmonella bacteraemia: epidemiology, clinical characteristics and its' association with severe immunosuppression. Ann Clin Microbiol Antimicrob. 2009;8:15.
- [CrossRef] [PubMed] [Google Scholar]
- Global burden of invasive nontyphoidal Salmonella disease, 2010 (1) Emerg Infect Dis. 2015;21:941-949.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing incidence of invasive nontyphoidal Salmonella infections in Queensland, Australia, 2007-2016. PLoS Negl Trop Dis. 2019;13:e0007187.
- [CrossRef] [PubMed] [Google Scholar]
- Non-typhoidal Salmonella rates in febrile children at sites in five Asian countries. Trop Med Int Health. 2010;15:960-963.
- [CrossRef] [PubMed] [Google Scholar]
- Presentation of lifethreatening invasive nontyphoidal Salmonella disease in Malawian children: a prospective observational study. PLoS Negl Trop Dis. 2017;11:e0006027.
- [CrossRef] [PubMed] [Google Scholar]
- Extraintestinal focal infections in adults with Salmonella enterica serotype Choleraesuis bacter emia. J Microbiol Immunol Infect. 2007;40:240-247.
- [Google Scholar]
- Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev. 2004;17:311-322.
- [CrossRef] [PubMed] [Google Scholar]
- Bacteremia caused by Salmonella enterica serotype Choleraesuis in Taiwan. J Microbiol Immunol Infect. 2006;39:358-365.
- [Google Scholar]
- Epidemics of invasive Salmonella enterica serovar enteritidis and Senterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963-969.
- [CrossRef] [PubMed] [Google Scholar]
- Extraintestinal infections caused by nontyphoidal Salmonella from a tertiary care center in India. J Lab Physicians. 2018;10:401-405.
- [CrossRef] [PubMed] [Google Scholar]
- First case report of blood and urine cultures positive bacteraemia by Salmonella enterica serotype Choleraesuis from India. JMM Case Rep. 2014;1:1-4.
- [CrossRef] [Google Scholar]
- Molecular characterization of cephalosporin and fluoroquinolone resistant Salmonella Choleraesuis isolated from patients with systemic Salmonellosis in Thailand. Antibiotics (Basel). 2021;10:844.
- [CrossRef] [PubMed] [Google Scholar]
- Non-typhoidal Salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633-1641.
- [CrossRef] [PubMed] [Google Scholar]
- Decreasing clinical response of quinolones in the treatment of enteric fever. Indian J Med Sci. 2001;55:189-194.
- [Google Scholar]
- Rationale of azithromycin prescribing practices for enteric fever in India. Indian J Med Microbiol. 2012;30:30-33.
- [CrossRef] [PubMed] [Google Scholar]






