Translate this page into:
Clinico-Hematological Profile of Acute Leukemia Cases in Bihar: A Multiparameter Study in a Tertiary-Care Hospital
Address for correspondence: Iffat Jamal, MBBS, MD, Department of Hematology, Indira Gandhi Institute of Medical Sciences, Patna, 800014, Bihar, India (e-mail: iffatjamal111@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
The actual incidence and demographic profile of hematological malignancies are unknown in Bihar because of lack of population-based cancer registry (PBCR) data and specialized tertiary cancer center facilities. The objective of this study was to estimate the prevalence, clinico-hematological profile and subtyping of acute leukemia cases by retrospective medical records.
Materials and Methods
A retrospective study was conducted in the Department of Hematology, Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India, over 2 years from July 2019 to June 2021. A total of 176 cases with relevant clinical features and hematological findings were involved in the study. Medical records were studied and data were retrieved.
Statistical Analysis
Data were recorded and analyzed using SPSS version 25.
Results
A total of 176 cases with relevant clinical features and hematological findings were involved in the study. Acute myeloid leukemia (AML) was most prevalent (52.8%), followed by acute lymphoblastic leukemia (ALL) (34.1%) and unclassified acute leukemia cases (13.1%). Flow cytometry correlation was available in 150 cases. The ratio of males (62.5%) to females (37.5%) is 1.6:1. There was statistically significant difference in physical examination findings between AML and ALL patients. Splenomegaly, lymphadenopathy, and sternal tenderness were more often seen in ALL than in AML patients (p < 0.05). Pallor was more significantly associated with AML than with ALL patients (p < 0.05). Anemia and leucocytosis were found to be significantly associated with acute leukemia patients (p < 0.000).
Conclusion
AML M2 was the most common subtype of AML, and B-ALL was the most common subtype of ALL cases.
Keywords
hematological
malignancies
acute leukemia
myeloid
lymphoid
Introduction
Leukemia is a group of hematological malignancy in which there is an uncontrolled, unregulated, and rapid proliferation of leukemic cells resulting in replacement of normal hematopoietic cells by abnormal proliferating cells in bone marrow and spilling over peripheral blood as well.[1]
It accounts a major proportion of hematopoietic neoplasms that are diagnosed worldwide. According to GLOBOCAN 2020, the worldwide estimates of cancer incidence and mortality for developing countries in 2020 revealed 269,503 and 205,016 new cases of leukemia in males and females, respectively.[2] Of these, the estimated deaths were 176,000 and 132,000 in males and females, respectively. Approximately 23,660 deaths (13,900 males and 9,760 females) in the United States are expected to be attributed to leukemia in 2021.[3] Hence, timely diagnosis and initiation of treatment in initial stages is required for complete remission.
Leukemias are classified into two broad categories: acute and chronic. Acute leukemia is further subdivided into myeloid and lymphoid. In adults, acute myeloid leukemia (AML) accounts for 80 to 90% of cases of acute leukemias.[4] Acute lymphoblastic leukemia (ALL), common in childhood, comprises 12% of all leukemias.[5] Incidence rises again in the sixth decade, but this peak age is not commonly seen in developing countries.
Immunophenotyping by flow cytometry (FCM) is an essential aid for accurately diagnosing and prognosticating acute leukemias. It serves various purposes, mainly identifying and differentiating neoplastic population from normal population, defining number of neoplastic cells with their phenotypes, typing and subtyping of leukemias, and minimal residual disease analysis.[6]
To the best of our knowledge, there is a lack of literature regarding distribution and pattern of acute leukemia cases in Bihar. Population-based cancer registry (PBCR) is still lacking in major eastern states of India including most populous Uttar Pradesh and Bihar which can keep a track and notify regarding the prevalence and incidence of various types of cancer including leukemias.[7] Because of population growth, changing dietary habits, increasing tobacco consumption, and exposure to arsenic in the Gangetic belt, Bihar is anticipated to bear greater cancer burden, including hematolymphoid malignancies.
Objective
To estimate the prevalence and clinico-hematological profile of acute leukemia cases.
To study immunophenotypic profile in patients with acute leukemia.
Materials and Methods
A retrospective study was conducted in the Department of Hematology, Indira Gandhi Institute of Medical Sciences, Patna, over a period of 2 years from July 2019 to June 2021 and it was approved by the Institutional Ethical and Scientific committee. A total of 176 cases with relevant clinical features and hematological findings were involved in the study. A predesigned proforma was made in which patient characteristics were entered. Medical records were studied and data were collected.
Inclusion and Exclusion Criteria
All patients presenting with relevant clinical features and hematological findings suggestive of acute leukemia and those with myelodysplastic syndrome transformed to acute leukemia were included in the study, whereas all those patients who were on prior chemotherapy or radiotherapy were excluded from the study.
The required quantity of venous blood will be collected in EDTA vials. The collected blood will be analyzed by using fully automated analyzer (SIEMENS ADVIA 2120i) having six parts from which large unstained cell count will be estimated and subsequently peripheral blood smears will be prepared in such cases on glass slides and stained with Leishman's stain. Special stains like MPO and PAS will be done in all cases. Bone marrow aspiration will be done and slides stained with Leishman's stain will be evaluated and its findings will be correlated with immunophenotyping (done by BD FACS Canto, based on the principle of hydrodynamic focusing) and cytogenetics for further confirmation. Data were recorded and analyzed using Statistical Package for Social Sciences version 25 (SPSS version 25). Chi-square test and Fisher's exact test were applied to test the association of qualitative data and Student's t-test was applied to test the association of quantitative data. Results were recorded as frequencies, means ± standard deviations, and p-values. For all purposes, a p-value of less than 0.05 (95% confidence level) was considered as the criteria of significance.
Results
Out of 176 cases, 93 cases (52.8%) were of AML and 60 cases (34.1%) were of ALL. The remaining 23 cases (13.1%) could not be classified into any type of acute leukemia morphologically on bone marrow aspirate. FCM correlation was available in 150 cases. Due to financial constraint, immunophenotyping was not available for the remaining 26 cases. On FCM out of 150 cases, 92 cases turned out to be AML and 56 cases were ALL. Two cases were diagnosed as mixed phenotypic acute leukemia. There was concordance between morphological and immunophenotypic diagnosis in 95% of cases.
Overall 62.5% (n = 110) of patients suffering from acute leukemias were males, while 37.5% (n = 66) were females. ALL in males accounted for 61.7% of cases, while in females, it accounted for 38.3% of cases. 37.6% (n = 35) of AML cases were females, while 62.4% (n = 58) were male patients (►Table 1).
| Male | Female | Total | ||
|---|---|---|---|---|
| Age | 0–10 y | 16 (57.1%) | 12 (42.8%) | 28 (15.9%) |
| 11–20 y | 22 (61.1%) | 14 (38.8%) | 36 (20.4%) | |
| 21–30 y | 14 (63.6%) | 8 (36.3%) | 22 (12.5%) | |
| 31–40 y | 28 (65.1%) | 15 (34.8%) | 43 (24.4%) | |
| 41–50 y | 15 (57.6%) | 11 (42.3%) | 26 (14.7%) | |
| 51–60 y | 9 (75.0%) | 3 (25.0%) | 12 (6.8%) | |
| 61–70 y | 4 (57.1%) | 3 (42.8%) | 7 (3.9%) | |
| 71–80 y | 2 (100.0%) | 0 (0.0%) | 2 (1.1%) | |
| Residency | Urban | 41 (65.0%) | 22 (34.9%) | 63 (35.7%) |
| Rural | 69 (61.0%) | 44 (38.9%) | 113 (64.2%) | |
| Occupation | Student | 21 (60.0%) | 14 (40.0%) | 35 (19.8%) |
| Farmer | 28 (70.0%) | 12 (30.0%) | 40 (22.7%) | |
| Government employee | 11 (61.1%) | 7 (38.8%) | 18 (10.2%) | |
| Private | 7 (63.6%) | 4 (36.3%) | 11 (6.25%) | |
| Unexplained | 43 (59.7%) | 29 (40.2%) | 72 (40.9%) | |
Most of the study participants, 43 (24.4%), were within the age range of 31 to 40 years with a mean age of 28.8 ± 17.3 years. Among patients suffering from ALL, the majority of patients were of pediatric age group (85.9%), while for AML, the majority were adults (94.6%). For ALL, the mean age was 12.4 ± 10.1 years (ranging from 3 to 52 years). For AML, the mean age was 37.8 ± 13.3 years (ranging from 11 to 80 years).
Of the study participants, 113 (64.2%) were rural residents. From a total of 176 acute leukemia patients, 104 (59.0%) have provided their occupational status, whereas 72 (40.9%) did not comply with any occupation. Of these patients, farmer and students have the highest figures, 22.7 and 19.8%, respectively, while the least frequent were private workers (11, 6.25%; ►Table 1).
Fever was the most common presenting complaint followed by pallor in both AML and ALL. There was statistically significant difference in physical examination findings between AML and ALL patients. Splenomegaly, lymphadenopathy, and sternal tenderness were more often seen in ALL than in AML patients (p < 0.05). Pallor was more significantly associated with AML than with ALL patients (p < 0.05; ►Table 2, ►Fig. 1).
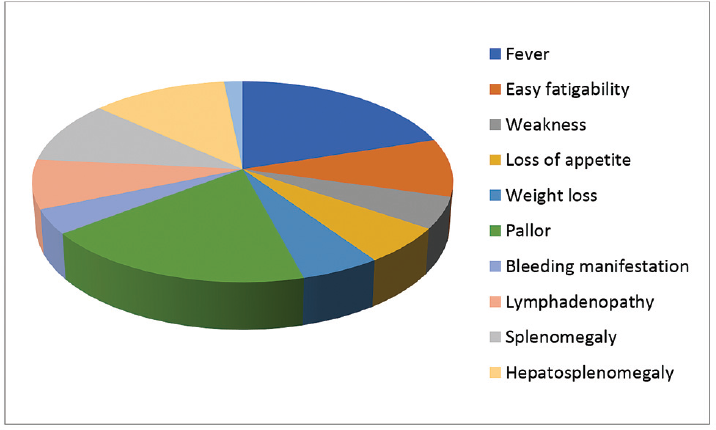
- Major clinical manifestations of the study population.
| Clinical features | AML | ALL | UC | Chi-square test | p-Value |
|---|---|---|---|---|---|
| Fever | 86 (53.1%) | 54 (33.3%) | 22 (13.6%) | 0.775 | 0.679 |
| Easy fatigability | 37 (49.3%) | 29 (38.7%) | 9 (12.0%) | 1.221 | 0.543 |
| Weakness | 19 (46.3%) | 18 (43.9%) | 4 (9.8%) | 2.385 | 0.303 |
| Loss of appetite | 29 (60.4%) | 14 (29.2%) | 5 (10.4%) | 1.541 | 0.463 |
| Weight loss | 22 (50.0%) | 16 (36.4%) | 6 (13.6%) | 0.193 | 0.908 |
| Pallor | 85 (55.9%) | 52 (34.2%) | 15 (9.9%) | 10.739 | 0.005 |
| Bleeding manifestations | 19 (59.4%) | 9 (28.1%) | 4 (12.5%) | 0.734 | 0.693 |
| Lymphadenopathy | 22 (34.4%) | 37 (57.8%) | 5 (7.8%) | 25.216 | 0.000 |
| Splenomegaly | 34 (42.0%) | 36 (44.4%) | 11 (13.6%) | 8.102 | 0.017 |
| Hepatosplenomegaly | 47 (49.5%) | 36 (37.9%) | 12 (12.6%) | 1.349 | 0.509 |
| Sternal tenderness | 3 (23.1%) | 9 (69.2%) | 1 (7.7%) | 7.748 | 0.021 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; UC, unclassified.
Out of 176 cases, 158 (89.7%) patients presented with leucocytosis. Only 7 cases (3.9%) had normal leukocyte count and 11 cases (6.2%) had leucopenia (►Table 3).
| Parameter | Range | Number of cases | % |
|---|---|---|---|
| Total WBC count/mm3 | < 4,000 | 11 | 6.2 |
| 4,000–11,000 | 07 | 3.9 | |
| 11,000–50,000 | 28 | 15.9 | |
| 50,000–100,000 | 72 | 40.9 | |
| 100,000–200,000 | 45 | 25.5 | |
| > 200,000 | 13 | 7.3 | |
| Hemoglobin (g/dL) | < 6 | 54 | 35.5 |
| 6.1–10 | 90 | 59.2 | |
| 10.1–12 | 08 | 5.2 | |
| Platelet count/mm3 | < 20,000 | 13 | 7.3 |
| 20,000–50,000 | 53 | 30.1 | |
| 50,000–100,000 | 101 | 57.3 | |
| > 100,000 | 09 | 5.1 | |
| Blast % on peripheral blood smear | < 20 | 15 | 8.5 |
| 21–89 | 104 | 59.0 | |
| 90 or above | 57 | 32.3 |
Abbreviation: WBC, white blood cell.
Anemia was detected in 100% of acute leukemia patients. There was severe degree of anemia in 35.5% of cases (hemoglobin < 6 g/dL), and 59.2% had moderate degree of anemia (hemoglobin: 6.1–10 g/dL), while 5.2% cases had mild anemia. No case showed percent of hemoglobin more than 12 g. Mean hemoglobin in AML is 7.2 ± 2.0 gm/dL, while mean hemoglobin in ALL is 7.4 ± 1.6 gm/dL (►Table 3).
A total of 101 patients (57.3%) showed platelets count between 50,000 and 100,000/mm3, 53 cases (30.1%) showed 20,000 and 50,000/mm3, 13 cases (7.3%) showed platelets count less than 20,000/mm3, and only 9 cases (5.1%) showed platelets count more than 100,000/mm3 (►Table 3).
Fifteen cases (8.5%) showed blasts less than 20%, 104 cases (59.0%) showed blasts between 21 and 89%, and 57 cases (32.3%) showed blasts more than 90%. Mean blast percent was 66.5 ± 27.4 in AML and 60.5 ± 29.6 in ALL (►Table 3).
FCM was done in 150 (85.2%) patients of which 56 had ALL, 92 had AML, and 2 was classified as mixed phenotypic acute leukemia (►Table 4). Among the remaining 14.8% of study population, FCM was not done because of financial constraints.
| Subtyping of AML on flow cytometry | No. of cases |
|---|---|
| AML M0 | 03 |
| AML M1 | 18 |
| AML M2 | 40 |
| AML M3 | 05 |
| AML M4 | 15 |
| AML M5 | 09 |
| AML M6 | 0 |
| AML M7 | 02 |
| Subtyping of ALL on flow cytometry | |
| B-ALL | 45 |
| T-ALL | 11 |
| Mixed phenotypic acute leukemia | 02 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia.
Cytogenetics was done in all five cases of AML M3 patients and PML RARA was found positive.
Discussion
Leukemias have varied presentation. As there are no proper cancer registry programs in Bihar, there is limited comprehensive assessment of clinical and laboratory profile of acute leukemia in this region. As compared with the West, lower age standardized incidence rates have been observed for most of the hematological malignancies.[8]
A total of 176 cases of acute leukemia were diagnosed on the basis of clinical course, laboratory data, and morphological features of leukemic cells. In this study, the AML-to-ALL ratio was approximately 1.5:1. The finding was consistent with the findings of Kassahun et al, Idris et al, and Jatav et al.[1,9,10] This study is a hospital-based study and only those patients who visited us have been included; hence, the findings are not externally valid.
In the present study, it was found that acute leukemias were more common in males, with a male (62.5%) to female (37.5%) ratio of 1.6:1 (AML vs. ALL: 62.4 vs. 37.6% and 61.7 vs. 38.3%, respectively). This is consistent with previous other studies.[9,11,12] Significant male preponderance reported in India can be partially explained by skewed gender selection of cases at the time of presentation rather than true gender disparity.[13]
In the present study, maximum incidence of acute leukemia was found in 31 to 40 years of age (24.4%), followed by 11 to 20 years of age (20.4%). Findings of our study were not exactly comparable to that of Shahab and Raziq, where maximum incidence was seen in 1 to 5 years of age group followed by over 35 years of age.[14] Kassahun et al reported maximum incidence of acute leukemia was seen among patients older than 50 years (35.5%).[1]
A majority of patients suffering from acute leukemia belong to rural background. The high preponderance may be because of varieties of chemical exposures such as pesticides, herbicides, and fertilizers used in agricultural activities which may result in genetic mutations leading to leukemogenesis.[15] Regarding the occupation distribution of patients who have AML, the highest proportion of 22.7% was recorded in patients who were farmers (p < 0.001).[15] On the other hand, ALL was more common in students (p < 0.001). This may be due to the age distribution that most of the patients in the age group of 0 to 20 years are students.
Clinical presentation of acute leukemia is very vague. In this study, most common presenting clinical feature was fever followed by pallor, hepatosplenomegaly, easy fatigability, and lymphadenopathy. These findings were consistent with several other studies.[9,11,14] This result is supported by the fact that the infiltration of leukemic cells in the bone marrow and their increased production will result in neutropenia (resulting in fever), anemia, and thrombocytopenia (bleeding manifestations).[16]
Statistically significant difference was found in physical examination findings of AML and ALL. Splenomegaly, lymphadenopathy, and sternal tenderness were more associated with ALL compared with AML (p < 0.05). Pallor was more associated with AML than with ALL patients (p < 0.05). These findings were consistent with previous literature.[9,14,17] These data support the idea that patients in our region go to hospitals when their condition has progressed to an advance stage.
In our study, anemia is the most common hematological abnormality followed by thrombocytopenia and leucocytosis which has also been exemplified by Rathee et al.[18] Maximum patients (59.0%) had hemoglobin of 6.1 to 10 g/dL followed by less than 6 g/dL in 35.5% of cases. Thrombocytopenia and leucocytosis were most common hematological abnormality in a study conducted by Preethi and Manisha et al.[19,20] Anemia and leucocytosis were found to be significantly associated with acute leukemia patients (p < 0.05).
In this study, median blast percentage in AML and ALL was 79 and 63%, respectively. Rathee et al found that the median blast proportion in AML was 45 and 38% in ALL.[18] In AML, Ghosh et al found mean values of 41.4% for peripheral blood blasts and 57.6% for bone marrow blasts.[21]
AML and ALL patients were further categorized into subtypes by using the FAB classification.[6]
On FCM out of 150 cases, 92 cases were AML and 56 cases were ALL. Two cases were diagnosed as mixed phenotypic acute leukemia. Most common subtype of AML in our study was AML M2, followed by AML M1 and AML M4. In ALL, most common subtype was B-ALL. Minimal screening panels recommended for immunophenotyping of acute leukemia include CD10, CD19, CD7, CD5, CD13, CD33, CD117, CD34, HLA-DR, and CD45.[6] This finding was consistent with the study conducted by Preethi and Ghosh et al.[19,21] According to Shahab and Raziq, AML-M4 was the most frequent subtype.[14]
There were several flaws in this study as it was a hospital-based retrospective research. The study is prone to selection bias and the findings of this study cannot be generalized.
Conclusion
In our study, AML was more common than ALL and males were frequently more involved than females. AML M2 was the most common subtype of AML found in our study. To develop our PBCR to know the future predictions of leukemia and for health surveillance, a detailed clinico-hematological study is utmost needed which is still lacking from our region that makes our study worth reporting.
Acknowledgment
We would like to thank study participants (patients) for their willingness to take part in the study and all data collectors for their active participation.
Conflict of Interest
None declared.
Funding
None.
References
- Prevalence of leukemia and associated factors among patients with abnormal hematological parameters in Jimma Medical Center, southwest Ethiopia: a cross-sectional study. Adv Hematol 2020:1-7.
- [CrossRef] [Google Scholar]
- Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(03):209-249.
- [Google Scholar]
- Accessed February 1, 2022 at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html
- WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2018.
- [Google Scholar]
- Acute leukemia in children: a review of the current Indian data. South Asian J Cancer. 2016;5(03):155-160.
- [CrossRef] [PubMed] [Google Scholar]
- Report of proceedings of the national meeting on “guidelines for immunophenotyping of hematolymphoid neoplasms by flow cytometry”. Indian J Pathol Microbiol. 2008;51(02):161-166.
- [CrossRef] [PubMed] [Google Scholar]
- Pediatric cancers in Bihar: a retrospective tertiary cancer center study. South Asian J Cancer. 2020;9(01):53-55.
- [CrossRef] [PubMed] [Google Scholar]
- An experience with sixty cases of haematological malignancies; a clinico haematological correlation. J Ayub Med Coll Abbottabad. 2004;16(04):51-54.
- [Google Scholar]
- Clinicopathological study of acute leukemia - a multiparameter study. Int J Contemp Med Res. 2016;3(11):3117-3120.
- [Google Scholar]
- Pattern of occurrence of leukemia at a teaching hospital in eastern region of Nepal - a six year study. JNMA J Nepal Med Assoc. 2009;48(173):35-40.
- [CrossRef] [Google Scholar]
- Pattern of leukaemias in a tertiary care hospital – a 5 years retrospective study of 103 cases. Ind Med Gaz 2013:175-180.
- [Google Scholar]
- Childhood cancer registrations in the developing world: still more boys than girls. Int J Cancer. 2001;91(03):402-406.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentations of acute leukemia. J Coll Physicians Surg Pak. 2014;24(07):472-476.
- [Google Scholar]
- Positive association of farm or rural residence with acute myeloid leukemia incidence in a cohort of older women. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2446-2448.
- [CrossRef] [PubMed] [Google Scholar]
- ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. 2016;6(07):e441.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and laboratory characteristics of patients with leukaemia in South-Nigeria. The Internet J Oncology. 2009;7(02):10-13.
- [Google Scholar]
- Incidence of acute and chronic forms of leukemia in Haryana. Int J Pharm Pharm Sci. 2014;6(02):323-325.
- [Google Scholar]
- Clinico-hematological study of acute myeloid leukemias. J Clin Diagn Res. 2014;8(04):14-17.
- [Google Scholar]
- Childhood acute lymphoblastic leukemia: Indian experience. Indian J Med Paediatr Oncol. 2004;25:12-18.
- [Google Scholar]
- Haematologic and immunophenotypic profile of acute myeloid leukemia: an experience of Tata Memorial Hospital. Indian J Cancer. 2003;40(02):71-76.
- [CrossRef] [PubMed] [Google Scholar]






