Translate this page into:
Clinicomycological Study of Dermatophytosis in South India
Address for correspondence: Dr. Lakshmi Vasantha Poluri, E-mail: plvasantha@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Dermatophytic infections are commonly encountered a problem and constitute more than 50% of cases in dermatology outpatient departments. Diagnosis of these infections requires the proper use of laboratory methods.
Objectives:
This study was conducted to know the etiology of dermatophytosis in patients attending Tertiary Care Level Hospital in South India and to compare the efficacy of Sabouraud's dextrose agar (SDA) with actidione and dermatophyte test medium (DTM) in isolating and identifying dermatophytes.
Materials and Methods:
A total of 110 samples which included 101 skin samples and 9 hair samples from clinically suspected dermatophytosis were collected. Direct microscopy by KOH and culture on SDA with actidione and DTM were done.
Results:
Of 110 samples collected, 58.18% were KOH positive for fungal filaments and 56.36% were culture positive for dermatophytes. More number of cases were observed between age groups of 21–40 years. Males were more affected compared to females. Tinea corporis was the common clinical presentation observed (40%). Trichophyton rubrum (58.06%) was the predominant isolate recovered in all clinical presentations but Trichophyton violaceum was the most common isolate in tinea capitis. All culture positives were grown on both SDA with actidione and DTM. Appearance of growth was earlier on DTM that is, within 10 days compared to SDA with actidione where growth started appearing only after 10 days. This is statistically significant P < 0.0001 (χ2 = 71.6). Species level identification on primary isolation was possible when grown on SDA with actidione and it was not possible with the growth on DTM on primary isolation.
Conclusion:
DTM is a good screening medium in laboratory diagnosis of dermatophytosis when compared to SDA with actidione. But DTM is inferior to SDA with actidione in identification of dermatophyte species.
Keywords
Dermatophte test medium
dermatophytosis
Sabouraud's dextrose agar with actidione
INTRODUCTION
Dermatophytosis is a common clinical entity characterized by the infection of keratinized tissues such as skin, hair, and nails. This is caused by a group of fungi called dermatophytes. Trichophyton, Microsporum, and Epidermophyton are the genera implicated to cause dermatophytoses. These dermatophytes are closely related filamentous fungi and cause the disease by virtue of their unique ability to degrade keratin and invade the skin and its appendages.[1] Dermatophytic infections are of major importance, as they are widespread and cause discomfort. Reactions to dermatophyte infection may range from mild to severe. The mildness and severity depend on a variety of factors such as the host reactions to the metabolic products of the fungus, the virulence of infecting species or particular strain, anatomical location of the infection and local environmental factors.
Dermatophytic infections are a common clinical problem encountered in more than 50% of patients attending the dermatology outpatient departments. Overcrowding, poor hygiene, low standards of living along with high humidity environments are contributing to the increased prevalence of these fungal infections. The present study was conducted to know the prevalence, etiology and common clinical presentations of dermatophytosis involving skin and hair. Predisposing factors favoring and laboratory methods for diagnosing dermatophytosis were also evaluated.
MATERIALS AND METHODS
A total of 110 samples which included 101 skin samples and 9 hair samples, from clinically suspected to have dermatophytosis were collected. A detailed history regarding age, sex, occupation, social status, duration of complaint and others were taken.
Collection of samples
Samples were collected after cleaning the affected surface with 70% alcohol. From skin lesions, scales were collected from erythematous growing margins of the lesion with a sterile blunt scalpel, and in case of tinea capitis, hairs were plucked with sterile surgical forceps. Samples were collected in sterilized Whatman filter paper envelope and transported to the microbiological laboratory. Fungal spores resist drying and remain viable for several weeks when stored in paper.
Direct microscopic examination
Direct examination of fungal elements from skin scales and hair samples was done by using 10% and 20% KOH mounts respectively.
Isolation by culture
All the samples were cultured on Sabouraud's dextrose agar (SDA) with gentamicin and cycloheximide (SDA with actidione) and dermatophyte test medium (DTM) (Hi-media). Samples were inoculated in two sets of these culture media. One set was incubated at 37°C and another set at 25°C in BOD incubator. Cultures were examined thrice weekly for the appearance of growth. Cultures were incubated for 1-month before discarding them as negative. Fungal growth was identified by colony morphology; pigment production and microscopic examination by tease mount technique in lactophenol cotton blue. Urease test and in-vitro hair perforation tests were also performed to differentiate Trichophyton rubrum and Trichophyton mentagrophytes when there was difficulty in identification by microscopic and macroscopic examination.[2]
RESULTS
In this study, 110 cases of clinically diagnosed dermatophytosis were studied. Males were more predominant 74 (67.27%) compared to females 36 (32.73%). Male: female ratio was 2:1. More number of cases were observed between age groups of 21–40 years 71 (64.54%) [Table 1]. Clinical presentations of dermatophytosis observed were tinea corporis 44 (40%), followed by T. corporis with cruris 28 (25.45%), tinea cruris 10 (9.09%), tinea capitis 9 (8.19%), tinea faciei 7 (6.36%), tinea manuum 6 (5.45%), tinea pedis 5 (4.55%) and tinea barbae 1 (0.91%). T. corporis and T. corporis with cruris were more common in the age groups of 21–40 years. Tinea capitis was more common in children < 10 years of age.

Fungal elements by KOH mount were observed in 64 (58.18%) and culture was positive in 62 (56.36%). Of 64 (58.18%) KOH positive cases 55 (85.9%) yielded growth in culture. Among KOH negative 46 (41.82%) cases, 7 (15.2%) were culture positive. Thirty-nine cases were negative by both KOH mount and culture.
Culture positivity was highest in T. corporis (38.71%) and no culture positivity was noted in tinea barbae and tinea pedis.
Dermatophytic species isolated were T. rubrum 58.06% [Figure 1], T. mentagrophytes 22.58% [Figure 2], Epidermophyton floccosum 6.45% [Figure 3], Trichophyton violaceum 6.54% [Figure 4], Trichophyton tonsurans 3.22% [Figure 5] and Trichophyton schoenleinii 3.22% [Figure 6].
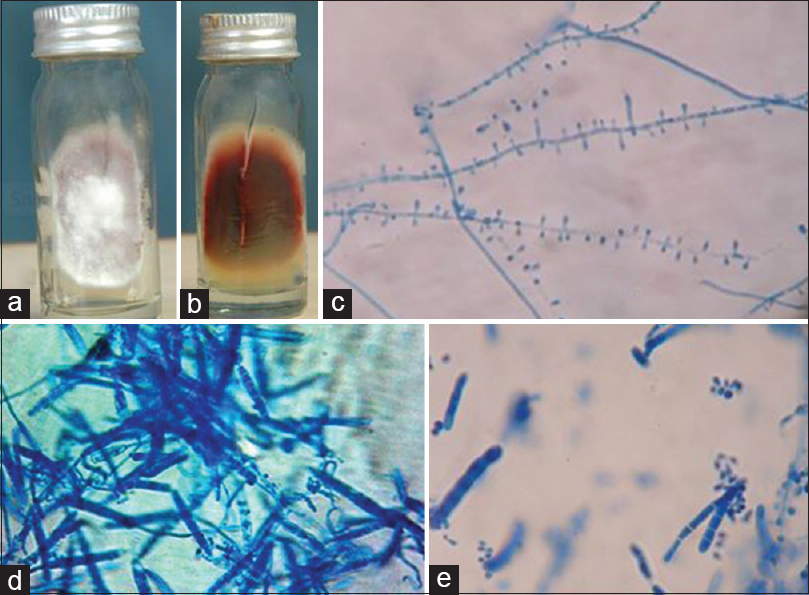
-
Trichophyton rubrum. (a) Growth on Sabouraud's dextrose agar (obverse). (b) Growth on Sabouraud's dextrose agar (reverse with pigmentation). (c) Lacto phenol cotton blue mount showing tear drop shaped microconidia (×400). (d and e) Lacto phenol cotton blue mount showing pencil shaped macroconidia (×400)
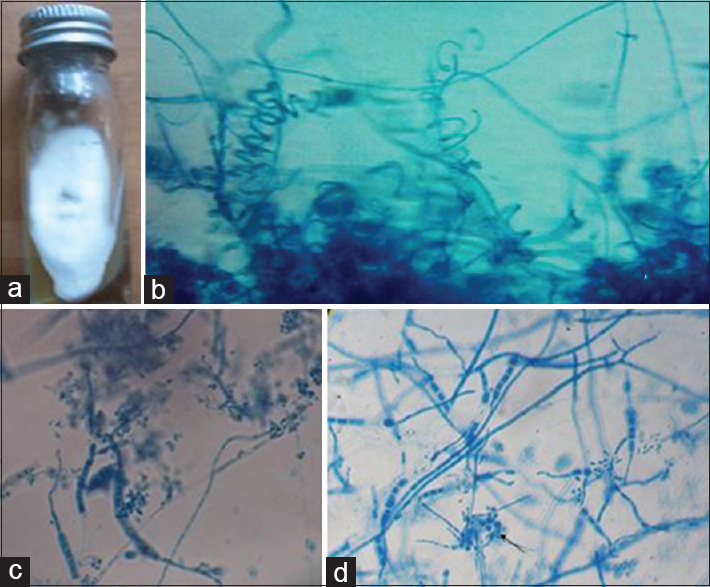
-
Trichophyton mentagrophytes. (a) Growth on Sabouraud's dextrose agar. (b) Lacto phenol cotton blue mount showing spiral hyphae (×400). (c) Lacto phenol cotton blue mount showing macroconidia (×400). (d) Lacto phenol cotton blue mount showing Nodular organ (×400)
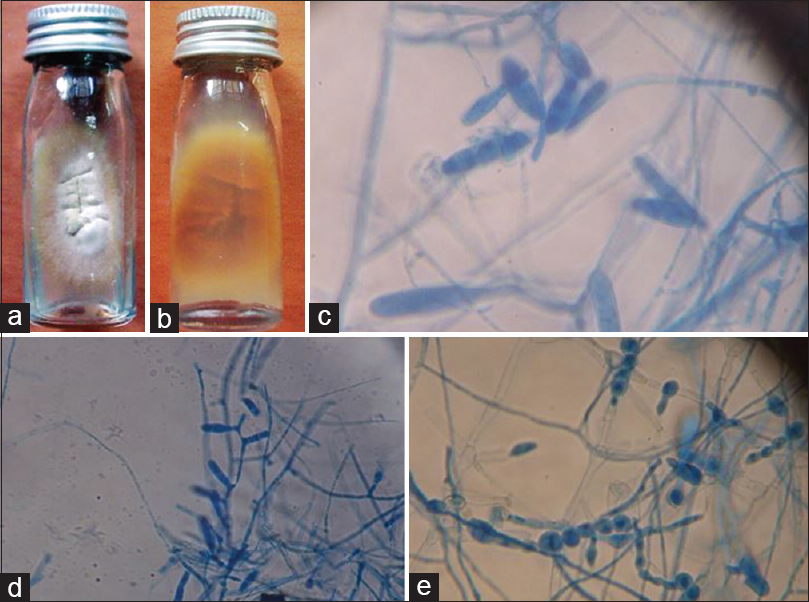
-
Epidermophyton floccosum. (a) Growth on Sabouraud's dextrose agar (obverse). (b) Growth on Sabouraud's dextrose agar (reverse). (c and d) Lacto phenol cotton blue mount showing club shaped macroconidia (×400). (e) Lacto phenol cotton blue mount showing characteristic chlamydospores (×400)
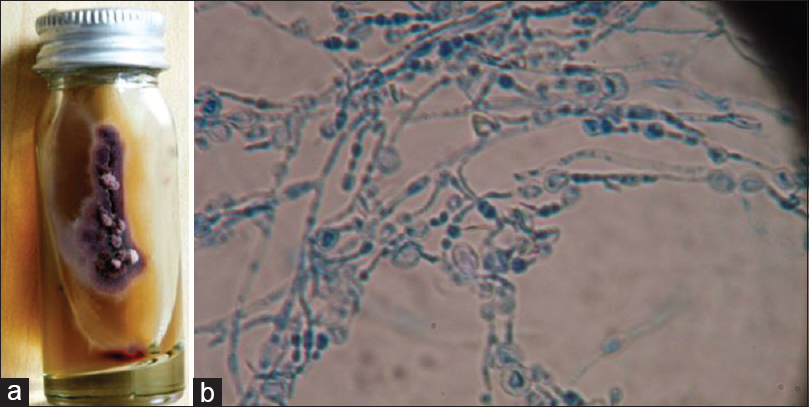
-
Trichophyton violaceum. (a) Growth on Sabouraud's dextrose agar (obverse with violet color pigmentation). (b) Lacto phenol cotton blue mount showing hyphae with chains of chlamydospores (×400)
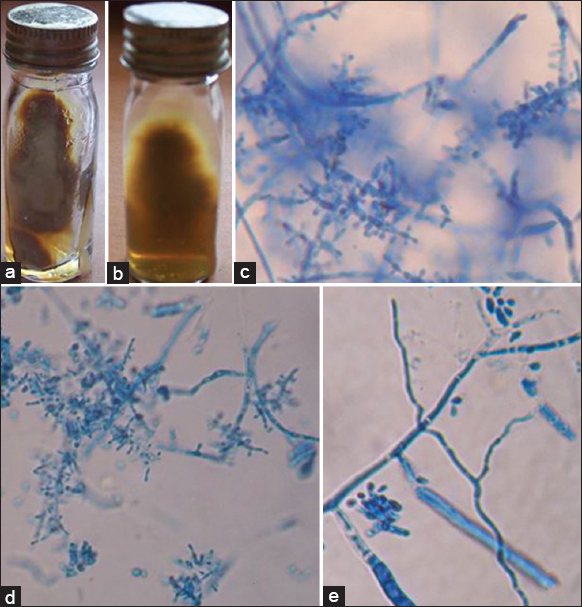
- Trichophyton tonsurans. (a) Growth on Sabouraud's dextrose agar (obverse). (b) Growth on Sabouraud's dextrose agar (reverse). (c) Lacto phenol cotton blue mount showing microconida (×400). (d) Lacto phenol cotton blue mount showing match stick like microconidia (×400). (e) lacto phenol cotton blue mount showing macroconidia (×400)
-
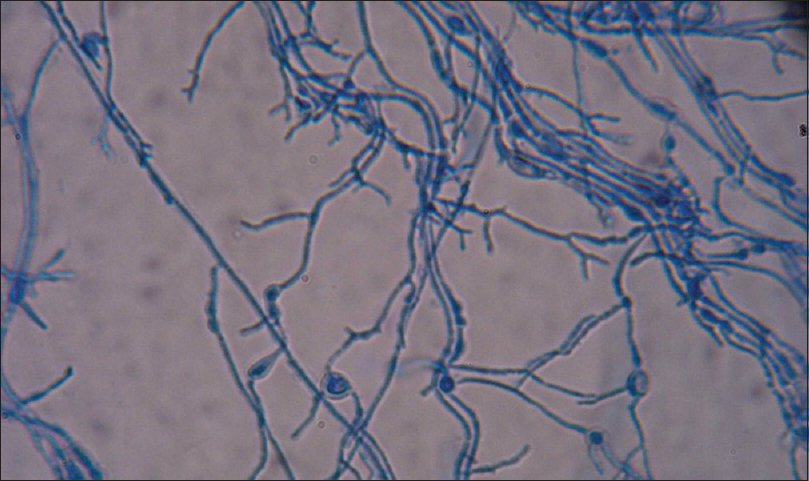
Trichophyton schoenleinii. Lacto phenol cotton blue mount showing antler hyphae and chlamydospores (×400)
T. rubrum was predominantly recovered in each clinical presentation but T. violaceum was the most common isolate in tinea capitis, followed by T. rubrum, T. tonsurans and T. schoenleinii [Table 2].

Comparison of growth pattern on Sabouraud's dextrose agar with actidione and dermatophyte test medium
All culture positives were grown both on SDA with actidione and DTM on primary isolation. On DTM first appearance of growth was within 10 days of inoculation for most of the isolates that is, 56 (90.32%) [Figure 7]. But, the appearance of growth was only after 10 days for most of the isolates when grown on SDA with actidione 57 (91.94%). Species level identification on primary isolation was possible for 42 (67.74%) of culture positives when grown on SDA with actidione. But, no culture positive was identified up to species level from DTM on primary isolation.
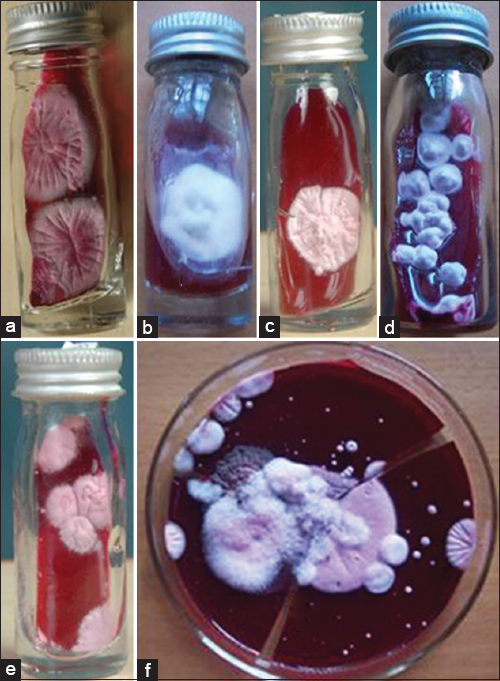
- Growth on dermatophyte test medium. (a) Trichophyton rubrum. (b) Trichophyton mentagrophytes. (c) Trichophyton tonsurans. (d) Epidermophyton floccosum. (e) Trichophyton schoenleinii. (f) Trichophyton violaceum
Among culture positive cases 19.35% were from rural area, and 80.65% were from urban area and 67.74% were from low socio-economic status, 30.65% were from middle socioeconomic status and 1.61% were from high socioeconomic status.
In this study, 8.06% of patients were diabetics, 6.45% were anemic, 3.23% of patients had a history of atopy and 1.61% were HIV positive.
Use of occlusive or synthetic fabric dressing regularly was there in 40.32% of patients and 32.25% of patients were in contact with soil regularly because of their occupation.
DISCUSSION
In this study, highest incidence of dermatophytosis was observed in the age group of 21–40 years and in males. This may be due to greater physical activity and increased sweating in this age group favoring the growth of dermatophytes. This was in correlation with other studies.[3456]
T. corporis, followed by T. corporis with cruris were common clinical presentations of dermatophytosis in the present study which was in correlation with other studies from India.[3567] T. capitis was more common in children below the age group of 10 years, which was also observed in some studies.[89]
T. rubrum was the most common dermatophyte to cause all clinical types of dermatophytoses followed by T. mentagrophytes. This was in correlation with other studies.[3567] Chronic nature of infection and adaptation to human is mainly responsible for predominance of T. rubrum to cause dermatophytosis.[10] T. violaceum was predominant species to cause tinea capitis, followed by T. rubrum, T. tonsurans and T. schoenleinii. E. floccosum mainly caused tinea cruris with corporis followed by T. cruris. Microsporum species were not isolated in the present study.
In the present study, KOH positivity was 58.18% and culture positivity was 56.36%. Culture positivity was more in KOH positive cases 85.9% compared to KOH negative cases 15.2%. This difference is statistically significant χ2 = 55.29; P < 0.001, this shows that direct microscopy by KOH mount is a good screening test in the laboratory diagnosis of dermatophytosis.
Efficacy of SDA with actidione and DTM in isolation of dermatophytes is equal in the present study. This was in correlation with the study of Singh and Beena who reported that 96.55% of culture positives were grown on SDA and 98.3% of culture positives were grown on DTM.[11]
But dermatophytes grow earlier on DTM compared to SDA with actidione. In the present study even though all 62 culture positive samples yielded growth both on SDA with actidione and DTM on primary isolation, appearance of growth was earlier on DTM that is, within 10 days (90.32%) compared to SDA with actidione (8.06%). This difference is statistically significant P < 0.0001 (χ2 = 71.6). This was reported in some studies.[9]
For species level identification of dermatophytes, SDA with actidione is preferable compared to DTM. In the present study, species level identification was possible for 67.74% of isolates on primary isolation on SDA with actidione. But species level identification was not possible with the growth on DTM on primary isolation, as conidial production was low on DTM, which is required for identification. Moreover, it was not possible to observe pigment production on DTM. For identification of growth on DTM, always it was required to subculture further on to SDA with actidione.
Dermatophytic infections were more common in people living in urban areas (80.65%) compared to rural areas (19.35%). This may be because the study included patients attending the outpatient department of dermatology of Tertiary Care Teaching Hospital, where most of the people were from the urban area. High incidence of dermatophytic infections was observed in the low socioeconomic group of people (67.74%). This is because of unhygienic living conditions, overcrowding, illiteracy and poor nutrition among them. This is in correlation with the study of Ranganathan et al.[12] Daily wage workers (35.48%) and students (33.87%) were predominantly affected compared to others. This could be because of increased physical activity and hot environmental conditions which results in increased sweating and excessive moisture favoring the growth of dermatophytes. This is in correlation with the study of Prasad et al.[13] In the present study systemic predisposing factors like diabetes (8.06%), anemia (6.45%), atopy (3.23%), and HIV (1.61%) were observed which are in correlation with other authors.[14] Local predisposing factors like occlusive or synthetic fabric dressing in 40.32% and contact with soil regularly because of their occupation in 32.25% were noted. These were also observed in other studies.[4613]
CONCLUSION
Dermatophytosis is the commonly encountered fungal infection in developing countries like India. Diagnosis of these infections requires proper laboratory aid. DTM is a good screening medium in laboratory diagnosis of dermatophytosis compared to SDA with actidione. But, DTM is not a preferable medium for identification of dermatophytes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Color Atlas and Textbook of Diagnostic Microbiology. (8th ed). Philadelphia: JB Lippincott; 1997.
- [Google Scholar]
- Scenario of chronic dermatophytosis: An Indian study. Mycopathologia. 1997;140:129-35.
- [Google Scholar]
- Profile of dermatophyte infections in Baroda. Indian J Dermatol Venereol Leprol. 2003;69:281-3.
- [Google Scholar]
- Clinico-mycological study of dermatophytosis in Calicut. Indian J Dermatol Venereol Leprol. 2002;68:259-61.
- [Google Scholar]
- Clinicomycological study of dermatophytosis in Bijapur. Indian J Med Microbiol. 2004;22:273-4.
- [Google Scholar]
- Epidemiology of dermatophytosis in and around Tiruchirappalli, Tamilnadu, India. Asian Pac J Trop Dis. 2012;2:286-9.
- [Google Scholar]
- Mycological study of dermatophytosis in rural population. Ann Biol Res. 2011;2:88-93.
- [Google Scholar]
- Epidemiology of the dermatophytoses, source of infection, modes of transmission of epidemicity. Ann N Y Acad Sci. 1960;89:69-99.
- [Google Scholar]
- Comparative study of different microscopic techniques and culture media for the isolation of dermatophytes. Indian J Med Microbiol. 2003;21:21-4.
- [Google Scholar]
- Effect of socio-economic status on the prevalence of dermatophytosis in Madras. Indian J Dermatol Venereol Leprol. 1995;61:16-8.
- [Google Scholar]
- A study of chronic dermatophyte infection in a rural hospital. Indian J Dermatol Venereol Leprol. 2005;71:129-30.
- [Google Scholar]
- Mycological study of dermatophytosis in 100 clinical samples of skin hair and nail. Int J Pharm Pharm Sci. 2013;5:763-5.
- [Google Scholar]





