Translate this page into:
Cluster of differentiation 4+ T-cell counts and human immunodeficiency virus-1 viral load in patients coinfected with hepatitis B virus and hepatitis C virus
Address for correspondence: Dr. Bharti Malhotra, Department of Microbiology and Immunology, Advance Research Laboratory, SMS Medical College, Jaipur, Rajasthan, India. E-mail: drbhartimalhotra@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Coinfections of human immunodeficiency virus (HIV) with hepatitis viruses may affect the progress of disease and response to therapy.
OBJECTIVES:
To study the incidence of hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfections in HIV-positive patients and their influence on HIV-1 viral load and cluster of differentiation 4+ (CD4+) T-cell counts.
MATERIALS AND METHODS:
This pilot study was done on 179 HIV-positive patients attending antiretroviral therapy (ART) centre. Their blood samples were tested for HIV-1 viral load, CD4+ T-cell counts, hepatitis B surface antigen, anti-HCV antibodies, HBV DNA and HCV RNA polymerase chain reaction.
RESULTS:
Among the 179 patients, 7.82% (14/179) were coinfected with HBV and 4.46% (8/179) with HCV. Median CD4+ T-cell count of HIV monoinfected patients was 200 cells/μl and viral load was 1.67 log10 copies/μl. Median CD4+ T-cell counts of 193 cells/μl for HBV (P = 0.230) and 197 cells/μl for HCV (P = 0.610) coinfected patients were similar to that of HIV monoinfected patients. Viral load was higher in both HBV and HCV infected patients but statistically significant only for HCV (P = 0.017). Increase in CD4+ T-cell counts and decrease in HIV-1 viral load in coinfected patients on 2 years of ART were lower than that in HIV monoinfected patients.
CONCLUSION:
HBV/HCV coinfected HIV patients had similar CD4+ T-cell counts as in HIV monoinfected patients, higher HIV viral load both in chemo-naive patients and in those on ART as compared to HIV monoinfected patients. However, this study needs to be done on a large scale to assess the impact of coinfection on CD4 count and HIV viral load with proper follow-up of patients every 6 months till at least 2 years.
Keywords
Cluster of differentiation 4+ T-cell count
coinfection
hepatitis B virus
hepatitis C virus
human immunodeficiency virus-1 viral load
Introduction
In India, approximately 21.17 lakhs (17.11 lakhs–26.49 lakhs) people are living with human immunodeficiency virus (HIV) as estimated in 2015 in a population of more than 1.3 billion.[1]
In the United States, among the HIV-positive patients, about 25% were coinfected with hepatitis C virus (HCV) and about 10% were coinfected with hepatitis B virus (HBV).[2] HBV endemicity in India is considered at an intermediate level with over 40 million HBV carriers.[3] As per the World Health Organization (WHO), India has 6–12 million people infected with HCV.
It is important to understand the impact of HBV and HCV coinfections on HIV viral load and cluster of differentiation 4 (CD4+) T-cell count in chemo-naive patients and in those on anti-retroviral treatment (ART). It has been shown in various studies that, following highly active ART (HAART), hepatitis-related liver disease mortality has significantly increased, and is the most common cause of non-AIDS-related deaths among HIV-positive patients.[4]
Although various authors have evaluated the short-term response to ART on HIV/HBV and HIV/HCV coinfected individuals, the impact of HBV and HCV on HIV treatment outcomes is not clear due to variable reports.[56789]
For proper management of HIV-positive patients, it is important to understand the impact of coinfection with HBV and HCV in terms of viral load and CD4+ T-lymphocyte counts in these patients versus the HIV-positive patients not having coinfection which is particularly important in South-East Asia region as it bears an estimated burden of 100 million chronic HBV and 50 million chronic HCV infections.[3]
Thus, the present study was undertaken with the objective to evaluate the impact of coinfection with HBV and HCV on HIV-1 viral load and CD4+ T-cell count among HIV-infected patients who are chemo-naive and on ART at a tertiary care center in western region of India.
Materials and Methods
One hundred and seventy-nine HIV-seropositive patients attending ART center, a tertiary care hospital, were enrolled in this pilot study carried out during 2013 (1 year). HIV-positive patients who had coinfections other than HBV or HCV such as Mycobacterium tuberculosis, candidiasis, and other fungal infections; viral infections such as herpes and cytomegalovirus; and other comorbidities were excluded from the present study. Detailed clinical history including signs, symptoms, list of medications, past history of opportunistic infections (OIs), and relevant laboratory reports and demographic characteristics of all known HIV-positive patients included in the study were recorded. Ethical clearance was obtained from institutional ethics committee and consent forms were filled by all patients.
CD4+ T-cell counts were estimated by BD FACS Calibur flow cytometer (Becton and Dickinson, San Jose, USA). HIV-1 viral load estimation was done using COBAS® TaqMan® 48 analyzer using COBAS® TaqMan® HIV-1 kit (Roche Diagnostics, Mannheim, Germany), which has a linear range of detection of 47 copies/ml to 107 copies/ml. Hepatitis B surface antigen (HBsAg) and anti-HCV antibodies were tested with a third-generation enzyme immunoassay (EIA) (J Mitra and Co Pvt., Ltd, New Delhi, India). Patients negative for HBsAg and anti-HCV antibodies by EIA were tested for the presence of HBV DNA/HCV RNA by a qualitative polymerase chain reaction (PCR) using primer sequences (5'-TTT CAC CTC TGC CTA ATC ATC TC-3' [sense primer] and 5'-TTT ACC TCT GCC TAA TCA TCT C-3' [anti-sense primer][10] for HBV DNA and outer primers 5'-CTG TGA GGA ACT ACT GCT T-3' [sense primer] and 5'-GGC TCA TGG TGC ACG GTC TAC GAG ACC TCC GG-3' [anti-sense primer]; inner primers-5'-TTC ACG CAG AAA GCG TCT AG-3' [sense primer] and 5'-CAC TCG CAA GCA CCC TAT-3' [anti-sense primer] for HCV RNA).[11] Amplification and detection were done using conventional PCR.[1011] Continuous variables were expressed as median (including interquartile range) and categorical variables as the number of cases (percentage). For measurement data, normal distribution of the measurement data was tested first. t-test (normal distribution) and Wilcoxon test were applied (nonnormal distribution) to analyze the differences among different groups of patients, i.e., CD4+ T-cell counts and HIV-1 viral load between HIV monoinfected and HIV-HCV or HIV-HBV coinfected patients. Tests were two sided, and P < 0.05 was considered statistically significant. Analyses were performed using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Out of the 179 patients, 14 (7.82%) were detected positive for HBV (5 by EIA and 9 by PCR) and 8 (4.46%) were detected positive for HCV (2 by EIA and 6 by PCR). Male:female ratio was 2.07:1 in HIV monoinfected group, 1.8:1 in HBV coinfected group, and 1.6:1 in HCV coinfected group. Median age of the patients in these three groups was 18, 35.5, and 29 years, respectively. In HIV monoinfected/HBV/HCV coinfected groups, 64.33%, 57.14%, and 60% of patients were confined to the WHO clinical stage I/II and 35.67%, 42.86%, and 30% to stage III/IV.
Overall, median CD4+ T-cell counts in HIV mono- and co-infected patients were similar. However, viral load of HIV-HBV and HIV-HCV coinfected patients was higher but statistically significant only for HCV coinfection (P = 0.017) [Table 1].

Median CD4+ T-cell counts were lower in chemo-naive HIV monoinfected patients and were higher in patients after 2 years of treatment (P = 0.133). Viral load of patients was significantly lower in patients after 2 years of ART as compared to chemo-naive patients (P = 0.032) [Table 2].
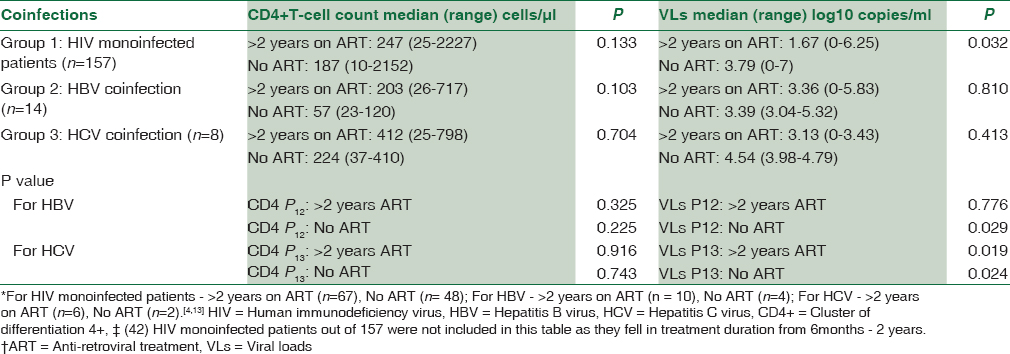
No significant difference was observed in CD4+ T-cell counts between both groups (HIV monoinfected vs. coinfected) of patients with and without ART. HIV-1 viral load in HBV and HCV coinfected chemo-naive patients was significantly higher than those of HIV monoinfected chemo-naive patients (for HBV, P = 0.029; HCV, P = 0.024). Patients on ART for more than 2 years also had higher HIV-1 viral load values in coinfected groups, but it was significant only for HCV coinfected group (for HBV, P = 0.776; HCV, P = 0.019) [Table 2].
Out of 14 HIV-HBV coinfected patients, chemo-naive patients were associated with lower median CD4+ T-cell counts as compared to patients on more than 2 years of ART (P = 0.103). Furthermore, the median HIV-1 viral load was higher in chemo-naive patients when these two groups were compared (P = 0.810) [Table 2].
Out of eight HIV-HCV infected patients, chemo-naive patients were associated with lower median CD4+ T-cell counts as compared to patients on more than 2 years on ART (P = 0.704). The median HIV load was higher in chemo-naive patients as compared to patients on 2 years of ART (P = 0.413) [Table 2]. However, both of them were statistically insignificant.
Discussion
Variable coinfection rates have been reported for HBV and HCV in HIV patients around the world, depending on the geographic area under study, risk groups, and the type of exposure involved. In the present study, 7.82% and 4.46% of HIV-infected patients were coinfected with HBV and HCV, respectively. Similar observations, i.e., HBV 6%–6.4% and HCV 2.1%–4.8%[112] have been reported from Chennai and 15% HBV and 8.3% HCV coinfection from Hyderabad.[13] Studies from North India have reported lower percentage of coinfection; Delhi reported 3.6%–7.28% HBV and 0.2%–2.2% HCV,[1415] Agra reported 9% HBV coinfections,[16] Kolkata reported 11.3% HBV and 1.9% HCV coinfection,[17] and Chhattisgarh reported 6% HBV and 2% HCV,[18] whereas very high prevalence rates have been reported from the USA, i.e., 30%–50%,[19] and 8.5% HIV-HBV and 2.8% HIV-HCV from western region of Saudi Arabia.[20]
In this study, we observed that CD4+ T-cell counts did not differ between HIV monoinfected and HBV/HCV coinfected patients. However, HIV-1 viral load was found to be higher in HBV and HCV coinfected group as compared to HIV monoinfected patients which was statistically significant for HCV coinfection (P = 0.078; P = 0.017). Similar findings were reported from Gondar, Africa, where mean CD4+ T-cell counts were 288 cells/mm3 in HIV monoinfection and slightly lower count (about 14–38 cells/mm3 lower) in HIV-HBV and HIV-HCV coinfected patients but not statistically significant.[21] On the contrary, previous studies from Jaipur and Chandigarh observed that HIV/HBV coinfected patients had significantly lower CD4 T-cell counts than the monoinfected group (P = 0.03).[2223] An American study also concluded that, compared with patients without coinfection, coinfected patients showed impaired CD4+ T-cell recovery, despite similar virological response to HIV-1 therapy.[8] Greivensen from Belgium also reported a lesser CD4 increase among HCV/HBV coinfected patients as compared to HIV monoinfected patients, but it was statistically significant only for HBV coinfection (P = 0.001).[24]
These variations may be due to differences in local epidemiology, prevalent genotypes of HIV/HBV/HCV, follow-up time, the viral loads of HBV and HCV, and whether patients were receiving any antiviral agents for HBV/HCV infection, population characteristics (in particular, the relative importance of intravenous drug users), etc.[24] Many a times, HIV patients are not aware of their HBV/HCV infection status; as a result, these infections remain unattended. In patients with HBV and HCV coinfections, HIV viral load may be a better marker to predict response to ART as increase in CD4+ T-cell counts may be similar to HIV monoinfected patients [Table 1].
In our study, in HBV and HCV coinfected groups as well as in HIV monoinfected patients, CD4+ T-cell counts were below 500 cells/μl despite remaining on ART for 2 years.
When HIV monoinfected group was compared to coinfected ones in terms of HIV-1 viral load in patients with and without ART, significant differences were obtained for chemo-naive patients only. Viral load of HIV monoinfected chemo-naive patients was lower than HCV coinfected (P = 0.024) but higher than HBV coinfected patients (P = 0.029). HIV-1 viral load of patients on ART for more than 2 years was higher in coinfected groups as compared to HIV monoinfected patients, but statistically significant only for HCV coinfected group (P = 0.019). HIV promotes hepatic fibrogenesis by production of reactive oxygen by hepatocytes through a Kappa-dependent pathway, this effect is enhanced in the presence of HCV.[25] Recent studies from Manipur, India,[6] Asia,[26] Switzerland,[27] and Australia[28] have postulated that patients coinfected with HIV/HBV/HCV appear to have a poorer response to HAART in terms of CD4+ T-cell count changes. A Chinese study also inferred similar results in HIV/HBV coinfected individuals at 48 months post-ART both in terms of CD4+ T-cell count and HIV-1 RNA suppression.[29] Studies from other parts of the world have reported variable results regarding CD4+ T-cell recovery and HIV-1 viral load suppression after ART. Many studies from India and around the world have reported that HBV/HCV coinfection did not affect CD4+ T-cell counts and HIV-1 viral load suppression after ART.[5679] It has been hypothesized that splenic sequestration of lymphocytes due to HBV-related hepatic fibrosis may be responsible for lower CD4+ T-cell recovery.[30] Many underlying comorbid conditions, other OIs, small sample size, and lack of compliance/adherence may affect these results.
Some patients enrolled in HIV care and prescribed ART do not attain an adequate virologic and immunologic response due to inconsistent retention in care, poor adherence, unfavorable pharmacokinetics, or unexplained biologic factors.[31] Failure of HIV-1 viral load to reach undetectable levels in patients on ART leads to faster disease progression.
To summarize our key findings, the primary outcome of our pilot study was that CD4+ T-cell counts were similar in coinfected patients versus monoinfected patients in both chemo-naïve patients and those on ART, which is controversial as compared to some of the other studies. HIV-1 viral load in HBV and HCV coinfection was significantly higher than those of HIV monoinfection in both chemo-naive patients and those on ART (HBV patients not significant statistically). Secondary outcome observed was that response to ART after 2 years in HIV monoinfected patients was not optimum, stress should be laid on patient compliance and adherence to ART.
There were many limitations in our study: the study design should have been better planned, prospective study is needed with larger sample size comparing CD4+ T-cell count and viral load in monoinfected and coinfected patients, follow-up of patients should have been done and data should have been collected before ART, at 6 months, 1 and 2 years of ART, and should have been analyzed accordingly. Test should have been done to identify occult HBV infection, correlate HBV and HCV viral load and genotypes with response to ART, correlate response with therapeutic drugs given, and test for other OIs too. Strength of our study was HIV viral load testing which has been done by only limited authors and we observed that HIV viral load may be better marker to evaluate response to ART in coinfection cases as only the CD4+ T-cell response may not be able to assess the same.
The National Aids Control Organization guidelines recommend the initiation of ART irrespective of CD4+ T-cell count values in HIV/HBV coinfected patients with documented evidence of chronic active hepatitis,[1] but there is no guideline for active screening of HIV patients for HBV and HCV coinfection and no additional care is taken in coinfected persons as regard to ART. There is a need to change this and active screening for HBV-HCV coinfection should be started as suggested by the CDC guidelines[2] and give aggressive combination therapy to treat HIV and coinfection. Treatment algorithms need to be evaluated planning right combination of drugs effective for HIV and HBV/HCV in case of coinfections to get optimal response. HBV vaccination needs to be implemented. Though there are many constraints such as cost, access to treatment, and laboratory investigations, simplified treatment and monitoring strategies need to be developed for resource-limited countries like ours.
Future prospective studies need to be planned to evaluate the extent and evolution of liver disease in coinfected individuals, effect of hepatitis treatment and ART on liver disease and the associated mortality, identify treatment algorithms for optimal care in HIV, HBV/HCV coinfection, associated risk factors, and the role of various genotypes and viral load of each virus. Effect of early initiation of ART and antiviral therapy for HBV/HCV should also be explored. Moreover, studies on the role of psychosocial aspects on prognosis and treatment outcome and other host factors such as compliance should also be taken into account when evaluating the impact of coinfection.
Conclusion
HIV-positive patients with HBV and HCV coinfections had lower CD4+ T-cell counts and higher viral loads in both chemo-naive and in patients on ART. Viral load can be a more sensitive marker to monitor response to ART. However, large-scale studies are needed. Screening for HBV and HCV in all HIV-positive patients is essential for timely detection and treatment of coinfection. Other factors such as comorbidities, individual immunological status, adherence, and relapse should also be monitored.
Financial support and sponsorship
This study was financially supported by the Indian Council of Medical research JRF – 2008 (33347) ICMR SRF (33347) and State DST project.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank all patients and technical staff in Advance Research Laboratory and ART centre, SMS Medical College and Hospital, Jaipur, Rajasthan, India.
References
- National AIDS Control Organization. Annual Report 2015-16. Available from: http://www.naco.gov.in/sites/default/files/Annual%20Report%202015.16_NACO.pdf
- Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. Recommendations from the Centres for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America; March. 2017. Available from: https://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf
- [Google Scholar]
- National Centre for Disease Control. Viral hepatitis in India, 2010 to 2013. Newsletter. 2014. :3. Available from: http://www.ncdc.gov.in/writereaddata/linkimages/NewsLtr0103_20146480274026.pdf
- [Google Scholar]
- Guidelines for Prevention and Management of Common Opportunistic Infections/Malignancies Among HIV-Infected Adult and Adolescent. NACO, Ministry of Health and Family Welfare, Government of India. Available from: http://www.Guidelines%20for%20Prevention%20and%20Management%20of%20common%20opportunistic%20infections.pdf
- [Google Scholar]
- Immunological and virological response to haart in HIV-1 patients co-infected with hepatitis B and C viruses. West Afr J Med. 2012;31:124-8.
- [Google Scholar]
- Clinical profile and response to first-line anti-retroviral therapy in Human Immunodeficiency Virus infected patients in Manipur. Int J Med Dent Sci. 2016;5:970-7.
- [Google Scholar]
- HCV RNA viral load is independent from CD4 cell count and plasma HIV RNA viral load in immunocompetent HIV-HCV co-infected patients: A 3-years follow-up study. AIDS Res Ther. 2014;11:21.
- [Google Scholar]
- CD4 recovery on antiretroviral therapy is associated with decreased progression to liver disease among hepatitis C virus-infected injecting drug users. Open Forum Infect Dis. 2015;2:ofv019.
- [Google Scholar]
- Baseline characteristics of HIV & amp; hepatitis B virus (HIV/HBV) co-infected patients from Kolkata, India. Indian J Med Res. 2016;143:636-42.
- [Google Scholar]
- Universal primers for HBV genome DNA amplification across subtypes: A case study for designing more effective viral primers. Virol J. 2007;4:92.
- [Google Scholar]
- Clinical & amp; molecular characterization of human TT virus in different liver diseases. Indian J Med Res. 2010;131:545-54.
- [Google Scholar]
- Hepatitis B or hepatitis C co-infection in individuals infected with human immunodeficiency virus and effect of anti-tuberculosis drugs on liver function. J Postgrad Med. 2006;52:92-6.
- [Google Scholar]
- Hepatitis B and/or C co-infection in HIV infected patients: A study in a tertiary care centre from South India. Indian J Med Res. 2013;138:950-4.
- [Google Scholar]
- Hepatitis B and C virus co-infections in human immunodeficiency virus positive North Indian patients. World J Gastroenterol. 2006;12:6879-83.
- [Google Scholar]
- Time trends of seroepidemiology of hepatitis C virus and hepatitis B virus coinfection in human immunodeficiency virus-infected patients in a super specialty hospital in New Delhi, India: 2012-2014. Indian J Sex Transm Dis. 2016;37:33-7.
- [Google Scholar]
- HIV, HBV, HCV, and syphilis co-infections among patients attending the STD clinics of district hospitals in Northern India. Int J Infect Dis. 2006;10:358-63.
- [Google Scholar]
- Characterization of treatment-naive HIV/HBV co-infected patients attending ART clinic of a tertiary healthcare centre in Eastern India. PLoS One. 2013;8:e73613.
- [Google Scholar]
- Prevalence of hepatitis B virus and hepatitis C virus co-infection in human immunodeficiency virus positive patients: A study from tribal area of central India. Int J Res Med Sci. 2015;3:2311-5.
- [Google Scholar]
- Veterans Affairs Hepatitis C Resource Center Program. National Hepatitis C Program Office. Management and treatment of hepatitis C virus infection in HIV-infected adults: Recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. Am J Gastroenterol. 2005;100:2338-54.
- [Google Scholar]
- Co-infection assessment in HBV, HCV, and HIV patients in Western Saudi Arabia. J Med Virol. 2016;88:1545-51.
- [Google Scholar]
- HBV and HCV seroprevalence and their correlation with CD4 cells and liver enzymes among HIV positive individuals at university of Gondar teaching hospital, Northwest Ethiopia. Virol J. 2013;10:171.
- [Google Scholar]
- Hepatitis B virus seroprevalence and its correlation with CD4 cells and liver enzymes among human immunodeficiency virus positive individuals at a tertiary care hospital in North-West India. Int J Appl Basic Med Res. 2015;5:36-40.
- [Google Scholar]
- Study of T-lymphocyte subpopulation in HBsAg-positive pregnant women. Acta Virol. 1993;37:459-65.
- [Google Scholar]
- Hepatitis B and C co-Infection among HIV-Infected adults while on antiretroviral treatment: Long-term survival, CD4+T cell count recovery and antiretroviral toxicity in Cambodia. Plos One. 2014;9:e88552.
- [Google Scholar]
- Hepatitis C in human immunodeficiency virus co-infected individuals: Is this still a “special population”? World J Hepatol. 2015;28(7):936-52.
- [Google Scholar]
- Hepatitis B and C co-infection in HIV patients from the TREAT Asia HIV observational database: Analysis of risk factors and survival. PLoS One. 2016;11:e0150512.
- [Google Scholar]
- Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: The Swiss HIV cohort study. Lancet. 2000;356:1800-5.
- [Google Scholar]
- HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med. 2003;4:241-9.
- [Google Scholar]
- Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014;28:29-34.
- [Google Scholar]
- Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013;208:1454-8.
- [Google Scholar]
- AIDS-defining opportunistic illnesses in US patients, 1994-2007: A cohort study. AIDS. 2010;24:1549-59.
- [Google Scholar]





