Translate this page into:
Comparison of Bone Mineral Density, T-Scores and Serum Zinc between Diabetic and Non Diabetic Postmenopausal Women with Osteoporosis
Address for correspondence: Dr. Priyanka Ramappa Siddapur, E-mail: priyankasiddapur@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Postmenopausal osteoporosis is a public health problem. Diabetics are at increased risk of osteoporosis-related fractures. Zinc (Zn) has a role in collagen metabolism, and its levels are altered in diabetes.
Aims:
The aim was to compare bone mineral density (BMD), T-score and serum Zn between diabetic and nondiabetic postmenopausal women with osteoporosis to see if they influence increased fracture risk in diabetes.
Settings and Design:
It is a cross.sectional study conducted at Department of Biochemistry, Jawaharlal Nehru Medical College, Belgaum.
Materials and Methods:
Thirty type 2 diabetic and 30 age-matched (aged 45-75 years) nondiabetic Dual energy X-ray absorptiometry (DEXA) confirmed postmenopausal osteoporotics were included from January 2011 to March 2012. Serum Zn was analyzed by atomic absorption spectrophotometry.
Statistical Analysis Used:
Mean and standard deviation of the parameters of the two groups were computed and compared by unpaired Student's t-test. Relationship between variables was measured by Karl Pearson's correlation co-efficient. A statistical significance is set at 5% level of significance (P < 0.05).
Results:
T-score was significantly higher in diabetics compared with nondiabetics (−2.84 ± 0.42 vs. −3.22 ± 0.74) P < 0.05. BMD and serum Zn of diabetics showed a significant positive correlation with body mass index (BMI).
Conclusions:
Type 2 diabetic postmenopausal osteoporotics have a higher T-score than the nondiabetics. High BMI in type-2 diabetes mellitus (T2DM) may contribute to high BMD and may be a protective factor against zincuria. Increased fracture risk in T2DM could be due to other factors like poor bone quality due to hyperglycemia rather than BMD. Strict glycemic control is of paramount importance.
Keywords
Bone mineral density
diabetes
menopause
osteoporosis
serum zinc
T-score
INTRODUCTION
The word “osteoporosis” literally means “porous bone.”[1] By definition, Osteoporosis is a disease characterized by low bone mass and deterioration of bone microarchitecture with a consequent increase in bone fragility and hence fracture risk.[2] It has been called as a silent epidemic as bone loss is silent and progressive often asymptomatic until the first fracture occurs.[1] Dual X-ray absorptiometry has become the standard for measurement of low bone mineral density (BMD) associated with osteoporosis. The definition of Osteoporosis according to World Health Organization (WHO) is a fall in BMD 2.5 standard deviations (SDs) below the mean for young healthy adults of the same gender which is also referred to as a T-score of – 2.5.[3]
In their lifetime30-50% of women and 15-30% of men are at risk for osteoporosis and its related fractures all over the world.[4] Conservative estimates suggest that around 25 million of Indian population would be affected by 2015.[5] Women are predominantly affected by this disease.[1] Menopausal period is associated with increased bone loss due to estrogen deficiency and ageing.[3] Although osteoporosis has not been traditionally listed as a complication of diabetes, patients with Diabetes are at increased risk for this disease.[6] Osteoclasts, the bone resorbing cells, require glucose for energy. Hence, enhanced osteoclast activity may be seen in diabetes. Hyperglycemia also leads to glycation of various bone proteins including type I collagen, which may impair bone quality.[7]
Zinc (Zn) an essential trace element is necessary for normal collagen synthesis and mineralization of bone.[8] It acts as a cofactor for alkaline phosphatase, bone forming metalloenzyme.[9] Reduced serum or plasma Zn concentrations and increased urinary Zn excretion have been reported in women with osteoporosis.[10] Zn also plays an important role in insulin action with several investigators showing the perturbation of Zn metabolism in diabetics.[11] It has not been clearly elucidated whether Zn deficiency is a consequence of hyperglycemia or whether Zn deficiency contributes to the pathogenesis of diabetes.[12]
Studies have shown increased risk of fractures and bone abnormalities in diabetes mellitus patients.[67] Hence, this study is undertaken to compare the BMD, T-scores and serum Zn between diabetic and nondiabetic postmenopausal women with osteoporosis to see if they are altered in diabetes contributing to increased fracture risk in diabetes.
MATERIALS AND METHODS
The study is cross-sectional in design. It was conducted in Department of Biochemistry, KLES Dr. Prabhakar Kore Hospital and Medical Research Centre, Belgaum between January 2011 to March 2012. It comprised of 30 diabetic and age-matched 30 nondiabetic clinically, diagnostically confirmed cases of Postmenopausal Osteoporosis attending the orthopedic unit of KLES Dr. PrabhakarKore Hospital and Medical Research Centre, Belgaum. Permission to conduct the study was obtained from the Institutional ethics committee on human subject's research of Jawaharlal Nehru medical college, Belgaum. All the cases were evaluated and selected by simple random technique after fulfilling selection criteria. The cases of Osteoporosis reported to the Department of Orthopedics, KLES Dr. Prabhakar Kore Hospital and Medical Research Center were screened. After finding the suitability as per selection criteria, they were requested to participate in the study and briefed about the nature of the study and interventions used. A written informed consent was obtained. The consented patients were enrolled in the study. Further descriptive data of the participants like name, age, sex, detailed history, were obtained by interviewing the participants and were recorded on a predesigned and pretested proforma.
The women were characterized as postmenopausal if they had not menstruated for at least 12 months. The study population belonged to the age group of 45-75 years. Dual energy X-ray absorptiometry (DEXA) scan ‘T’ score <−2.5 according to the WHO criteria[3] were considered to be osteoporotic. The diabetic group comprised of known cases of diabetes with at least 1 month duration. Women were identified as nondiabetic based on fasting plasma glucose levels <100 mg/dL according to the WHO criteria for diagnosis of Diabetes.[13] Women with surgical menopause, hypertensives, renal disorders, smokers, alcoholics and those being treated with biphosphonates, calcitonin, anabolic steroids, hormone replacement therapy, calcium, Vitamin D previously at any point since the beginning of menopause were excluded from the study. Diabetic women taking insulin preparations containing Zn were excluded.
It was intended to include both Type 1 and Type 2 diabetic women. However, the diabetic women who met the inclusion and exclusion criteria in this study belonged to type 2 DM category (T2DM).
According to previous hospital records, the number of postmenopausal DEXA confirmed osteoporotic cases from Orthopedic department in KLE hospital has been around 80 cases/year. The number of Diabetic patients with DEXA confirmed Osteoporosis was unknown. For the study to be statistically significant minimum sample size of 30 Diabetic and 30 nondiabetic postmenopausal women with osteoporosis were taken.
Bone mineral density assessment
Bone mineral density of the patient was determined using whole body densitometer, DEXA Scan (GE Healthcare Lunar prodigy advance, scanner serial no. PA + 302343, software version– ENCORE 2008 version 12.2, Germany) in KLE hospital.
Bone mineral density values and T-scores of L1-L4 Lumbar spine were noted. Height and weight were measured at the time of DEXA measurement and body mass index (BMI) was calculated as the weight divided by the square of the height (kg/m2).
Collection and storage of the blood sample
Five milliliter of blood was collected in the fasting state in the morning before breakfast in a plain nonvacuum tube and was allowed to stand in room temperature till clot was formed. Serum was separated within 1 h of venipuncture by centrifuging the tubes at 3,000 r.p.m for 10 min. Serum was pipetted out into sterile Eppendorf tubes. Serum glucose was estimated immediately by Trinder's Method[14] using ERBA reagent kit in ERBA Chem 5 semiautoanalyzer in KLEs Charitable Hospital laboratory. All samples were stored in nonvacuum sterile tubes at −20°C till further analysis.
Zinc estimation
Serum Zn estimation of the stored samples was done using Atomic Absorption Spectrophotometer (AAS) Perkin Elmer Analyst 300, University Science Instrument Centre, Shivaji University, Kolhapur. Serum sample was diluted to 1:10 or 1:5 with deionized water. The dilution ratio was adjusted so that the concentrations fall within a suitable absorbance range.
Standard
Zinc stock solution provided by the manufacturer was diluted, and appropriate standards were prepared.
Blank
Deionized water was used as blank solution.
Analysis
Instrument was set in standard condition for the Zn analysis. Blank was aspirated first followed by a suitable standard and then the sample. Zn standard was read after every five samples. The readings were recorded in parts per million (p.p.m) and then converted to μg/dL.
Statistical analysis
The data were analyzed by SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.
Mean and SD of BMD, T-score and serum Zn of the two groups were computed and compared by unpaired Student's t-test. Results are expressed as Mean ± SD. Relationship between variables was measured by Karl Pearson's correlation co-efficient. A statistical significance is set at 5% level of significance (P < 0.05).
RESULTS
Bone mineral density values and T-scores in 30 type 2 diabetic and 30 age-matched nondiabetic postmenopausal women with osteoporosis were compared.
The data obtained from the study was compiled, tabulated and subjected to statistical analysis. As the two groups were age-matched, there was no significant difference in the mean age in both the groups (P = 0.9669). Diabetics had a significantly higher mean weight and BMI than nondiabetic participants. Diabetics had a higher mean BMD than nondiabetics, but it was not statistically significant (P = 0.0661). However, the T-scores were significantly higher in case of diabetic women compared to nondiabetics (P = 0.0177). There was no significant difference in the mean serum Zn levels between diabetics and nondiabetic postmenopausal women with osteoporosis (P = 0.1042) [Table 1].
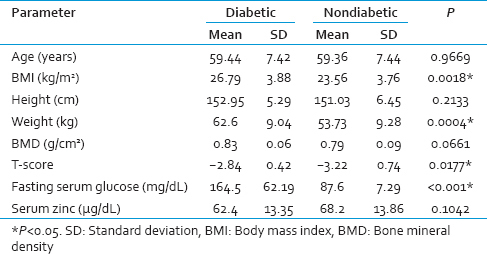
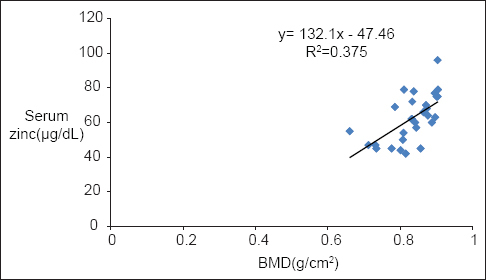
BMD levels showed a significant positive correlation with serum Zn in both diabetic P < 0.001 and nondiabetic participants P < 0.002 [Table 2, Graphs 1 and 2]. BMI of diabetics showed a significant positive correlation with Zn (P = 0.021) and BMD (P = 0.019) [Table 2, Graphs 3 and 4].
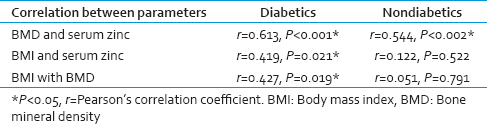
- Correlation of bone mineral density with serum zinc in diabetics
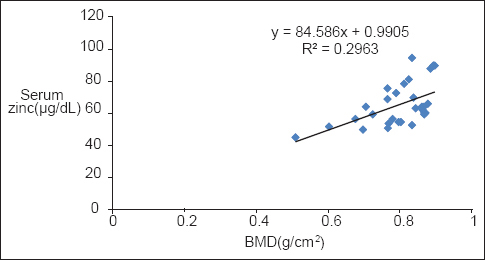
- Correlation of bone mineral density with serum zinc in nondiabetics
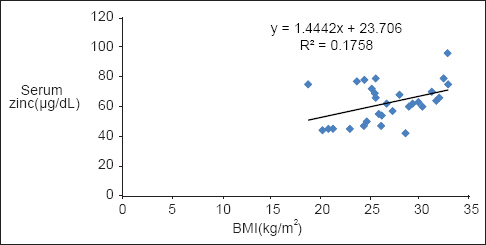
- Correlation of body mass index with serum zinc in diabetics
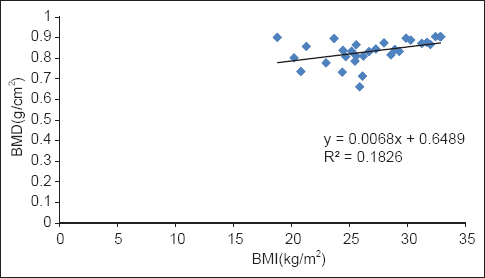
- Correlation of body mass index with bone mineral density in diabetics
DISCUSSION
30 Type 2 diabetic and 30 nondiabetic postmenopausal women with DEXA confirmed osteoporosis was chosen and their BMD levels, T-scores and serum Zn levels were compared.
This study found higher BMD levels in diabetic postmenopausal women with osteoporosis than nondiabetics though not statistically significant, while the T-scores were significantly higher in the diabetic group.
The pathogenic complexity of diabetes mellitus has led to conflicting results on bone involvement in this condition. One of the chronic complications of diabetes is bone loss. Hyperglycemia generates a high concentration of Advanced Glycation End products in collagen. They decrease bone strength, promote osteoblast apoptosis and increase osteoclast-mediated bone resorption. Several clinical trials have supported that new bone formation, bone microarchitecture and thus bone quality are altered in both types of diabetes resulting in increased fracture risk. T2DM is associated with low bone turnover, which slows bone loss that may lead to higher BMD levels.[15] Hyperinsulinemia is an important feature of T2DM. Insulin is structurally homologous to IGF-1 whose signaling pathway is crucial for bone acquisition. By interacting with the IGF-1 receptor present on osteoblasts, insulin shows an anabolic effect on bone. Hence, increased insulin levels in T2DM may also lead to high BMD levels.[16] Despite high BMD, there could be increased bone fragility in T2DM patients through the accumulation of fatigue damage.[15] Moreover, they have increased the risk of falling increasing the fracture risk.[16] Our study showed no significant difference in the mean serum Zn levels between the two groups though they were lower in Diabetics. This is in accordance with Zargar et al.[17] and Diwan et al.[18] who similarly found no significant difference in the serum Zn levels between type 2 Diabetics and controls. In contrast Emeribe et al.[19] found significantly higher serum Zn levels in type 2 diabetics compared to controls that they thought could be due to the imbalanced rate of absorption to excretion of Zn.
Urinary discharge of Zn has been shown to be increased in postmenopausal osteoporotic women and the degree of zincuria correlates with the severity of osteoporosis.[2021] Zincuria as a feature of diabetes has been demonstrated by several studies.[2223] Walter et al.[23] noted a six-fold increase in urinary Zn excretion in type 1 and type 2 diabetics compared with controls. Urinary Zn did not correlate significantly with plasma glucose concentrations, urinary glucose concentrations, HbA1c, proteinuria, or diuretic use.
Diabetic patients with renal diseases were excluded in this study. Hence, there must have been no significant zincuria in the chosen diabetic group. Moreover the T2DM participants were on regular oral hypoglycemic drugs. They were on one or the other sulfonylurea drugs. Sulfonylureas act by stimulating pancreatic β cells to secrete more insulin.[13] In addition, four participants were also on insulin therapy that did not contain Zn. Besides having anabolic effect on bone, insulin may reduce hyperzincuria accompanying diabetes.[22] Insulin and sulfonylureas which increase insulin secretion might have also been responsible for the insignificant difference in the serum Zn levels between diabetic women as compared to the nondiabetics in our study.
None of the diabetic patients was taking thiazolidinediones. These drugs activate PPARγ that induces adipogenesis over osteoblastogenesis in pluripotent cells.[15]
Obesity is another important feature of T2DM. Diabetic women in this study were obese and had a significantly higher BMI than the nondiabetics (P < 0.01). This is in accordance with Zargar et al.[17] The high BMI in this study was attributable to significantly higher weight of the diabetic women (P < 0.001). BMI of diabetics showed a significant positive correlation with Zn (P < 0.05). High BMI in T2DM may have a protective influence in maintaining serum Zn levels and to compensate for the decrease in Zn attributed to zincuria seen in DM.
Bone mineral density showed a significant positive correlation with serum Zn in both diabetics and nondiabetics. Serum Zn levels may serve to be important predictors of BMD.
Body mass index showed a significant positive correlation with BMD in the diabetic women (P < 0.05). This is in accordance with Brown et al.[24] Low BMI (<20) has been considered as an important risk factor for osteoporosis.[25] In obesity, there is increased conversion of androgens to estrogens by adipose tissue aromatase which have protective effects on bone.[19] Adipose tissue also releases a wide variety of adipokines that have been implicated in the regulation of bone remodeling. Body fatness may have an impact on the accuracy of DEXA based BMD measurement. However, such a measurement error is negligible.[16] No significant correlation between BMI and BMD in nondiabetics was found. There was not a major variation in the BMI among the nondiabetic participants, unlike diabetics. Most of them were in the normal BMI range. This could be the reason for the lack of correlation between BMI and BMD in nondiabetics.
In our study out of the 30 diabetic women, six were having FSG levels >200 mg/dL out of which three diabetic participants were recently diagnosed with diabetes with duration of 1 month. Severe Hyperglycemia in these women can be a confounding factor. The rest three diabetic women may not have been under strict glycemic control.
CONCLUSION
T-scores are significantly higher in type 2 diabetic postmenopausal women with osteoporosis compared with nondiabetics. Serum Zn levels are not significantly altered in T2DM postmenopausal women with osteoporosis when patients with overt nephropathy are excluded. Medications with sulfonylureas and/or insulin may reduce the zincuria that may accompany diabetes. Serum Zn levels may serve to be important predictors of BMD. High BMI in T2DM may contribute to high BMD and may be a protective factor against zincuria seen in diabetes mellitus. However to what extent these factors that have helped in maintaining serum Zn levels prevent diabetes-related fractures cannot be told as fractures in T2DM are attributable to poor bone quality rather than BMD. Strict glycemic control is recommended. More studies are required in this arena on the role of Zn in postmenopausal women with diabetic osteoporosis.
Limitations of the study
-
Both newly diagnosed and old cases of diabetes were included
-
Patients with overt diabetic nephropathy were excluded, but testing for microalbuminuria in diabetics that is an early predictor of nephropathy was not done.
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann Acad Med Singapore. 2008;37:564-7.
- [Google Scholar]
- Osteoporosis. In: Longo DL, ed. Harrison's Principles of Internal Medicine (18th ed). United States of America: McGraw-Hill Publishers; 2012. p. :3120-35.
- [Google Scholar]
- Profile of a menopause clinic in an urban population in Malaysia. Singapore Med J. 2000;41:431-5.
- [Google Scholar]
- Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317-28.
- [Google Scholar]
- Zinc intakes and plasma concentrations in men with osteoporosis: The Rancho Bernardo Study. Am J Clin Nutr. 2004;80:715-21.
- [Google Scholar]
- The status of trace elements after menopause: A comparative study. J Clin Diagn Res. 2011;5:795-7.
- [Google Scholar]
- Glycemic control of type 2 diabetic patients after short-term zinc supplementation. Nutr Res Pract. 2008;2:283-8.
- [Google Scholar]
- Blood Sugar lowering effect of zinc and multi vitamin/mineral supplementation is dependent on initial fasting blood glucose. J Diabetol. 2011;1:1-13.
- [Google Scholar]
- Diabetes mellitus. In: Longo DL, ed. Harrison's Principles of Internal Medicine (18thed). United States of America: McGraw-Hill Publishers; 2012. p. :2968-3003.
- [Google Scholar]
- Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24-7.
- [Google Scholar]
- Osteoporosis among patients with type 1 and type 2 diabetes. Diabetes Metab. 2008;34:193-205.
- [Google Scholar]
- Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur J Epidemiol. 2012;27:319-32.
- [Google Scholar]
- Copper, zinc, and magnesium levels in non-insulin dependent diabetes mellitus. Postgrad Med J. 1998;74:665-8.
- [Google Scholar]
- Serum zinc, chromium and magnesium levels in Type 2 diabetes. Int J Diabetes Dev Ctries. 2006;26:122-3.
- [Google Scholar]
- Serum chromium, zinc and testosterone levels in diabetics in university of Calabar Teaching Hospital Calabar Nigeria. J Biol Agric Healthc. 2012;2:93-100.
- [Google Scholar]
- Zinc intake and biochemical markers of bone turnover in type 1 diabetes. Diabetes Care. 2008;31:2279-80.
- [Google Scholar]
- Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. 1991;14:1050-6.
- [Google Scholar]
- Osteoporosis: An under-appreciated complication of diabetes. Clin Diabetes. 2004;22:10-20.
- [Google Scholar]
- In: Diagnosis and Treatment of Osteoporosis (8thed). Bloomington: Institute for Clinical Systems Improvement; 2013. p. :1-87.





