Translate this page into:
Comparison of screen-confirm versus screen-mix-confirm algorithms for lupus anticoagulant detection by dilute Russell’s viper venom time
*Corresponding author: Fatima Sharif, Department of Hematology, Shifa International Hospital, Islamabad, Pakistan. fatimasharif425@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharif F, Junaid A, Ashraf K. Comparison of screen-confirm versus screen-mix-confirm algorithms for lupus anticoagulant detection by dilute Russell’s viper venom time. J Lab Physicians. doi: 10.25259/JLP_110_2024
Abstract
Objectives
Lupus anticoagulant (LAC) is an antiphospholipid antibody associated with thromboembolism. According to the International Society on Thrombosis and Haemostasis, LAC measurement by Dilute Russell’s viper venom time (dRVVT) should follow a three-step procedure: “Screen-mix-confirm.” However, studies have raised concerns that the mixing step alters interpretation. Our aim is to assess the impact of a mixing study in LAC testing by dRVVT.
Materials and Methods
Patients undergoing LAC testing by dRVVT were prospectively enrolled from April 2021 to February 2022. Those who tested positive for the LA screen were tested by mixing and confirmation steps.
Statistical analysis
Data were analyzed using IBM® Statistical Package for the Social Sciences version 26. The chi-square test was used to assess whether the frequency of LAC positivity is significantly different with the addition of a mixing study. P < 0.05 was considered statistically significant.
Results
A total of 90 patients were included, with a mean age of 37 ± 14.2 years and a majority of females (n = 52, 57.8%). Similar results with both algorithms were seen in 76 (84.4%) samples. Among the 14 (15.6%) discrepant results, majority (10 out of 14) were weak positive for LAC by the two-step method while negative for LAC by the three-step method. The chi-square test revealed a significant difference in LAC positivity with and without a performance of mixing study (P < 0.001).
Conclusions
Interpretation of dRVVT with the inclusion of a mixing study leads to decreased frequency of LAC positivity. We recommend that a mixing step should not be used for weak positive LAC, to avoid discrepancies occurring due to the dilutional effect of mixing with normal plasma.
Keywords
Antiphospholipid syndrome
Laboratory diagnosis
Lupus coagulation inhibitor
INTRODUCTION
Lupus anticoagulant (LAC) is an antibody belonging to the class of antiphospholipid antibodies, which are associated with an increased risk of recurrent miscarriages and thromboembolic complications.[1] LAC can be measured through different methods such as activated partial thromboplastin time (aPTT), Kaolin Clotting time, Silica Clotting time (SCT), hexagonal phase phospholipid neutralization assay, and Dilute Russel Viper Venom Time (dRVVT).[2,3] At present, there is no single gold standard method to determine the presence of LAC; however, dRVVT is the preferred method in routine practice.[4,5]
Measurement of LAC by dRVVT includes a maximum of three steps. In the first step, or “screening step,” clotting time is measured with a reagent containing a limited concentration of phospholipids. A second “confirmatory” step involves measuring the clotting time with a reagent containing an excess of phospholipids. A possible third step is the inclusion of a “mixing” study, in which the patient’s plasma is mixed with an equal amount of normal plasma and the clotting time is then recorded.[6]
From the three steps of dRVVT, there is controversy in the current guidelines regarding the role of the mixing step. According to the International Society on Thrombosis and Haemostasis and the British Committee for Standards in Haematology, LAC measurement should be a three-step procedure in the order “screen-mix-confirm.”[7] Whereas the Clinical and Laboratory Standards Institute recommends that a mixing study does not always need to be performed and that if a mixing test is done, the testing order should be “screen-confirm-mix.”[8] The possibility of false negatives due to the mixing study is duly acknowledged by all the aforementioned guidelines.[9]
In this context, a study by Devreese and de Laat raised the concern of whether the mixing step should be included in LAC measurement or not.[10] They found that the frequency of LAC positivity was 88% using the two-step screen and confirm method, whereas the frequency of LAC positivity was reduced to 63.7% if a mixing study was considered as the third step in the interpretation of results.[10] Therefore, our study aims to determine the impact of the inclusion of a mixing study in the interpretation of LAC testing by the dRVVT method. Our purpose is to add to the existing, limited evidence in the literature, and build on to the foundation of current guidelines regarding LA testing.
MATERIALS AND METHODS
This prospective study was conducted in the Department of Hematology from April 2021 to February 2022. Patients whose LAC screening test by dRVVT is positive were consecutively included in the study for further testing through mixing and confirming tests. We excluded patients taking anticoagulant drugs as they are known to interfere with the dRVVT results, leading to inaccurate interpretation. According to the reference study by Devreese and de Laat, 170 out of 267 (63.7%) samples were LAC-positive when using the three-step method.[10] Hence, the sample size was calculated as follows, using the World Health Organization Sample size calculator: Confidence level - 95%; anticipated population proportion 63.7%; absolute precision required - 0.10; Sample size = 90.
Blood samples in 3.2% sodium citrate tubes are received regularly at Shifa International Hospital (SIH) for LAC testing. Samples undergo double centrifugation to collect platelet-poor plasma that is either processed immediately for LA testing or frozen at −15°C for testing later on after thawing. These samples undergo dRVVT on a CS-2500 automated coagulation analyzer (Siemens). Samples that test LA screen positive were then processed by mixing and confirmation methods. Screening steps were performed with LA1 reagent and confirmation steps with LA2 reagent, which has more quantity of phospholipid than LA1 (Siemens dRVVT kit). Equal volumes of pooled normal plasma were manually added to the patient’s plasma for the mixing step. Interpretation for all the blood samples was done through two algorithms: screen – confirm, versus screen – mix – confirm.[11]
Figure 1 summarizes the overall testing algorithm used in our study.
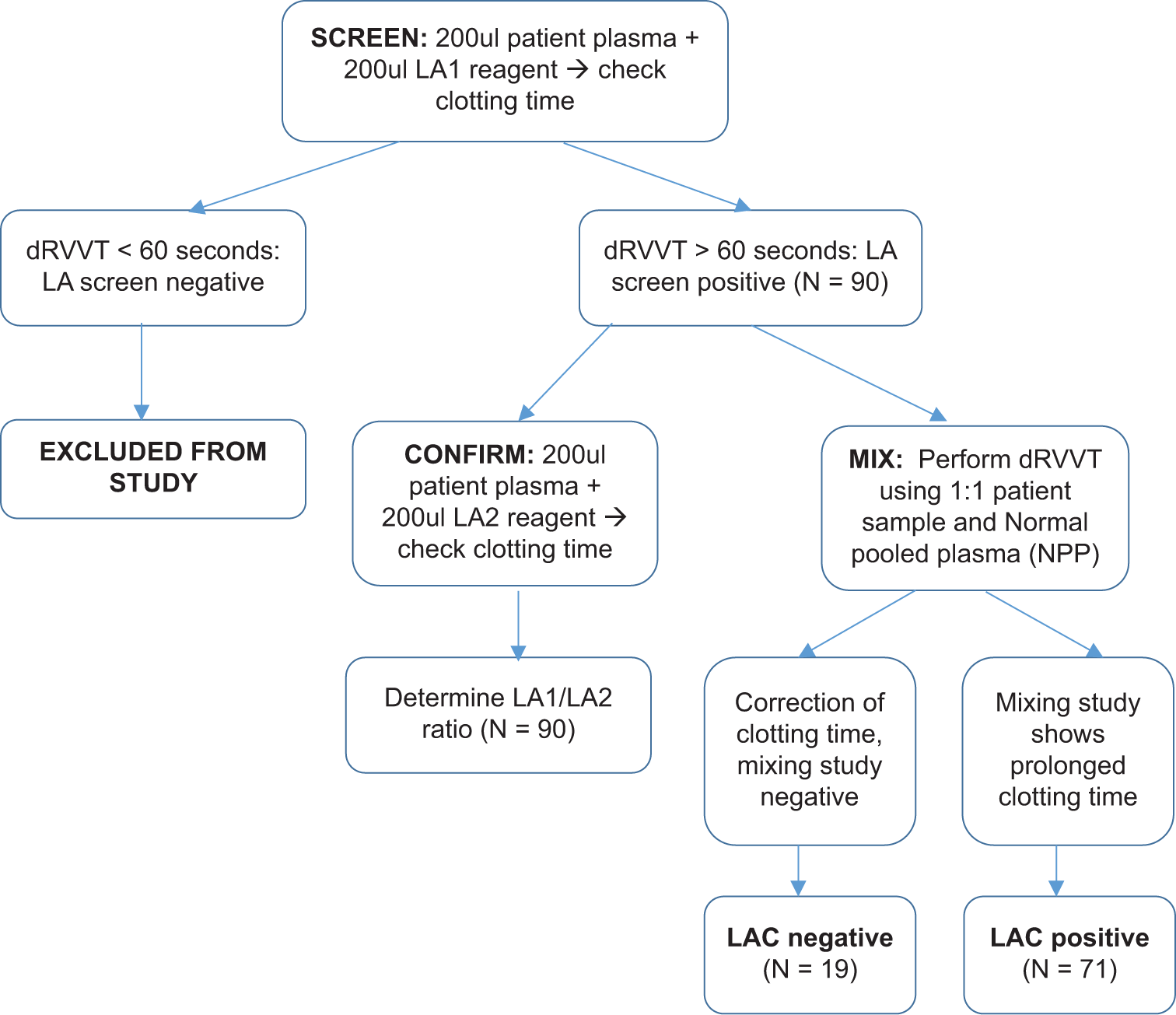
- Summary of methodology. dRVVT: dilute Russell’s viper venom time, LAC: Lupus anticoagulant, LA1 and LA2: Lupus anticoagulant reagents
The ratio of Screen and Confirm steps is calculated to determine the strength of LAC, interpreted as follows: <1.2: LAC negative, 1.2–1.5: Weak positive LAC, 1.5–2: Moderate positive, and >2 indicates the presence of strong positive LAC. These ratios were developed as part of our in-house evaluation of LAC testing and are comparable to the ratios used in other studies.[12,13]
Statistical analysis
Data were entered and analyzed using IBM® Statistical Package for the Social Sciences version 26. Mean and standard deviation were calculated for quantitative variables such as age. Frequencies and percentages were reported for qualitative variables, namely gender, indication for LAC testing, and LAC positivity. The chi-square test was used to determine whether the frequency of LAC positivity is significantly different between the two-step and three-step methods, with a P < 0.05 considered statistically significant.
This study was commenced after receiving ethical approval from the Institutional Review Board of SIH. Confidentiality has been maintained of all of the patients and none of the authors have any conflicts of interest to declare.
RESULTS
Among the 90 patients included in this study, 52 (57.8%) were female, while 38 (42.2%) were male. The mean age of the study participants was 37 ± 14.2 years. Thrombosis was the most common reason for undergoing LA testing in our patient population, seen in 45 (50%) of patients, followed by recurrent miscarriage due to suspected antiphospholipid syndrome in 25 (28%) patients.
On the ninety samples which tested screen positive for LAC, a confirmation step was performed. On confirmation step, 85 patients (94.4%) remained LAC positive while 5 patients (5.6%) tested negative for LAC. The majority of these patients had a moderate positive LAC. The interpretation of LAC testing by screening and confirmation steps is given below in Figure 2.
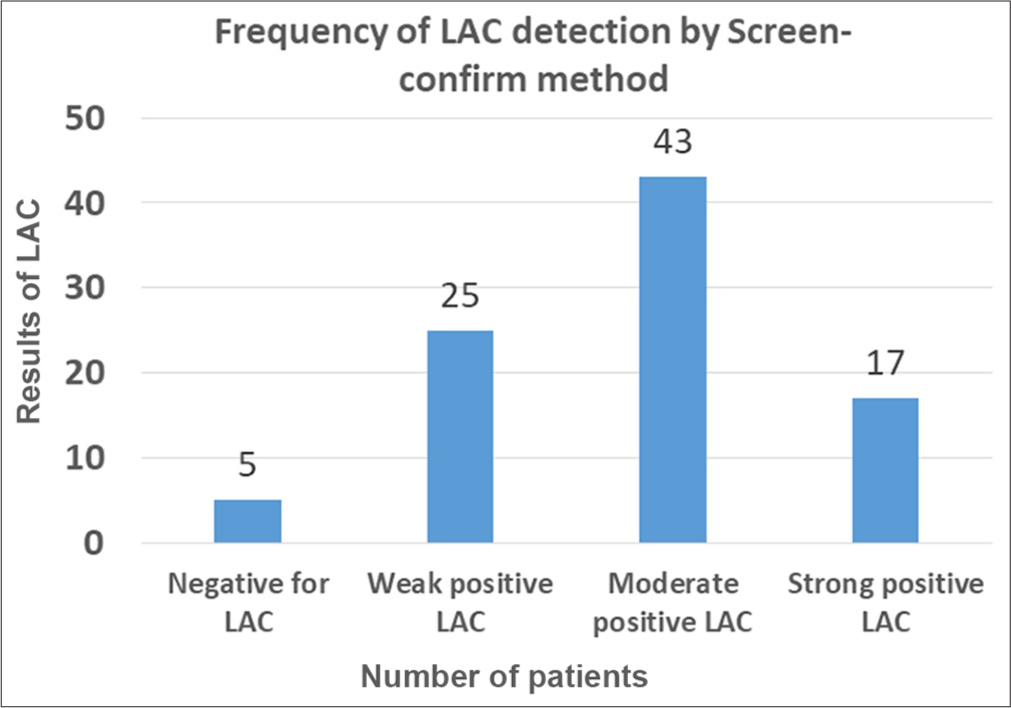
- Interpretation of lupus anticoagulant (LAC) testing by screening and confirmation steps. X axis shows results of LAC testing and Y axis number of patients
When a mixing study was performed on these samples after the screening and confirmation steps, 71 (78.9%) patients tested positive for LAC, while 19 (21.1%) were LAC negative.
Overall, it was found that 76 (84.4%) samples showed concordance with both methods of interpretation while 14 (15.6%) samples showed a discrepancy between two-step and three-step algorithms. These 14 samples were all positive for LAC by the Screen-confirm method but negative for LAC by the Screen-mix-confirm method. Among the 14 discrepant results, the two-step screening and confirmation technique showed weak LAC positivity in the majority of the cases (n = 10, 71% of discrepant cases), while the remaining 4 cases were moderately positive for LAC. There was no discrepancy among the cases that tested strong positive for LAC. The clinical history of these discrepant cases was either thrombosis or recurrent miscarriage, except in 2 out of 14 cases (14.3%) who underwent LAC testing as part of the pre-transplant thrombophilia screening protocol in patients with liver failure. A chi-square test was performed which showed that there is a significant difference in LAC detection with and without performance of a mixing study (P < 0.001) [Table 1].
| Screen-Mix-Confirm | p Value | |||
|---|---|---|---|---|
| LAC detected | LAC not detected | |||
| Screen-Confirm | LAC detected | 71 (78.9%) | 14 (15.6%) | <0.001 |
| LAC not detected | 0 (0%) | 5 (5.6%) | ||
LAC: Lupus anticoagulant
DISCUSSION
This study compared the frequency of LAC positivity by dRVVT when results were interpreted with and without the inclusion of a mixing study. Our results show that the addition of a mixing study leads to a decrease in the detection of LAC by Dilute Russell’s viper venom testing.
A similar study by Hong et al. investigated LAC positivity by two methods: dRVVT and SCT. They found that LA detected by the two-step method (screen and confirm) showed a greater risk of thrombosis as compared to when a mixing study was included in the diagnostic algorithm. The authors recommended that the two-step method without the addition of a mixing study should be the preferred technique to assess thrombotic risk.[14]
Another study by Chandrashekar found that when a mixing study was performed as part of the dRVVT, there were 83.8% false-negative results.[15] They recommended that a mixing study should only be used in conjunction with aPTT or dRVVT-based LAC detection methods in case of strong positive LAC or use of anticoagulant drugs. The results of our current study were concordant with these recommendations, as there were no discrepancies between the two-step and three-step methods in the case of strong positive LAC, defined as LA1:LA2 ratio >2.
Our study showed that the majority of discrepancies in LAC detection occurred when there was a weak positive LAC detected by the two-step method (defined as LA1:LA2 ratio of 1.2:1.5). However, clinically, there is no association between the “strength” of LA and risk of thrombosis.[16] Therefore, due to their clinical relevance, it is important to identify weak-positive cases of LAC as well, which may be missed using the three-step method.
An extensive review on laboratory diagnosis of LAC, including the use of mixing studies, was published in 2008 which concluded that due to a lack of standardization, the role of mixing studies in LAC detection is controversial, especially in the case of weak-positive LA.[17] Furthermore, weak-positive LAC may be missed if a mixing study is performed due to the dilutional effect of adding normal plasma to the test plasma.[18] This observation was also seen in our study, as the majority of discrepancies occurred in cases where weak-positive LAC was detected on screening and confirmation steps. In such a scenario, Ray et al. suggested that the clinical presentation should be considered when dealing with a negative mixing study so that those LACs that are false negative due to dilution are not missed.[19]
A recent study published in 2024 reported that the type of normal plasma used for mixing may also have a significant impact on the outcome of LAC testing. Biljak et al. prepared four types of mixing media: Standard human plasma, control plasma N, known patient plasma with normal coagulation values, and homemade normal pool plasma (NPP).[20] They found that when a mixing study was performed with NPP, the results of the mixing step during LAC detection were significantly different from the mixing test results using the other three types of plasma. However, in their study, LAC testing was done using an aPTT-based method. Such an investigation should be conducted with the dRVTT method in the future, to validate their findings across different methods and reagents, as these are the factors that may have a significant impact on test results.[21]
This study investigated important aspects of LAC testing and highlighted the impact of adding a mixing step during LA detection by dRVVT testing. A multicenter study with a larger sample size would be useful in verifying the findings observed in this current study.
CONCLUSIONS
Interpretation of dRVVT with the incorporation of a mixing study leads to reduced frequency of LAC positivity among patients who otherwise test positive by screen and confirm method. We recommend that while a mixing step cannot be eliminated, it should however not be used in case of weak-positive LAC, to avoid discrepancies that occur due to the dilutional effect of mixing with normal plasma.
Author’s contribution
FS: Study conception and design; data collection, data analysis, manuscript writing; AJ: Study conception, manuscript writing; KA: Manuscript writing.
Ethical approval
The research/study was approved by the Institutional Review Board at Shifa International Hospital, Pakistan, approval number 184-1004-2020, dated 8th June 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Antiphospholipid syndrome and recurrent miscarriage: A systematic review and meta-analysis. J Reprod Immunol. 2017;123:78-87.
- [CrossRef] [PubMed] [Google Scholar]
- Alternative assays to dRVVT and aPTT for lupus anticoagulant detection. Am J Hematol. 2020;95:992-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nine-test panel has superior sensitivity to detect antiphospholipid antibody syndrome in patients with or without SLE. Clin Immunol. 2020;214:108388.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus anticoagulant testing: Performance and practices by North American clinical laboratories. Am J Clin Pathol. 2010;134:764-73.
- [CrossRef] [PubMed] [Google Scholar]
- Testing for lupus anticoagulants. Semin Thromb Hemost. 2022;48:643-60.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus anticoagulant testing: Diluted russell viper venom time (dRVVT) Methods Mol Biol. 2017;1646:169-76.
- [CrossRef] [PubMed] [Google Scholar]
- Commonalities and contrasts in recent guidelines for lupus anticoagulant detection. Int J Lab Hematol. 2014;36:364-73.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory diagnosis of the lupus anticoagulant. Clin Lab Sci. 2017;30:7-14.
- [CrossRef] [Google Scholar]
- Recent guidelines and recommendations for laboratory detection of lupus anticoagulants. Semin Thromb Hemost. 2014;40:163-71.
- [CrossRef] [PubMed] [Google Scholar]
- Mixing studies in lupus anticoagulant testing are required at least in some type of samples. J Thromb Haemost. 2015;13:1475-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic challenges on the laboratory detection of lupus anticoagulant. Biomedicines. 2021;9:844.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus anticoagulant: A multicenter study for a standardized and harmonized reporting. Blood Coagul Fibrinolysis. 2016;27:176-84.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus anticoagulant interference in activated protein C resistance testing: In vitro phenomenon or in vivo pathophysiologic effect? Clin Appl Thromb Hemost. 2011;17:E190-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of the mixing test in laboratory diagnoses of lupus anticoagulant: The fate of the mixing test in integrated lupus anticoagulant test systems. Blood Coagul Fibrinolysis. 2012;23:739-44.
- [CrossRef] [PubMed] [Google Scholar]
- Dilute Russell's viper venom and activated partial thromboplastin time in lupus anticoagulant diagnosis: Is mixing essential? Blood Coagul Fibrinolysis. 2016;27:408-11.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory testing for lupus anticoagulants: Preexamination variables, mixing studies, and diagnostic criteria. Semin Thromb Hemost. 2008;34:380-8.
- [CrossRef] [PubMed] [Google Scholar]
- To mix or not to mix in lupus anticoagulant testing? That is the question. Semin Thromb Hemost. 2012;38:385-9.
- [CrossRef] [PubMed] [Google Scholar]
- To do or not to do mixing study in the era of integrated testing for lupus anticoagulant. Blood Coagul Fibrinolysis. 2024;35:223-4.
- [CrossRef] [PubMed] [Google Scholar]
- Mixing studies for lupus anticoagulant: Does it matter how we mix? Blood Coagul Fibrinolysis. 2024;35:129-32.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of different preanalytical conditions on results of lupus anticoagulant tests. Int J Lab Hematol. 2019;41:745-53.
- [CrossRef] [PubMed] [Google Scholar]






