Translate this page into:
Comparison of Ultrafast Papanicolaou Stain with the Standard Papanicolaou Stain in Body Fluids and Fine Needle Aspiration Specimens
Address for correspondence: Dr. Nasar Yousuf Alwahaibi, E-mail: nasar@squ.edu.om
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Most cytology laboratories in all Gulf countries including Oman, use the standard papanicolaou (PAP) method to stain various cytological specimens. The aim of this study was to investigate the possible application of ultrafast PAP (UF-PAP) method in cytology laboratory.
Materials and Methods:
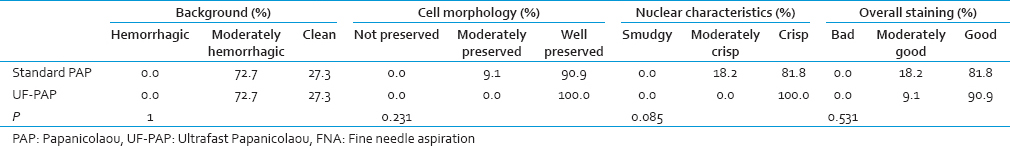
Samples from 46 patients containing 26 body fluids and 20 fine needle aspirations (FNAs) (9 thyroids and 11 breasts) were collected. Two air dried and two wet smears from each sample were prepared and stained by UF-PAP and the standard PAP stains, respectively. Background, nuclear staining, cell morphology, and overall staining were independently reviewed by two cytoscreeners.
Results:
In all cases of FNA, UF-PAP stain gave a good score for the background, nuclear staining, cell morphology, and overall staining when compared with the standard PAP method. Although the correct diagnosis was made in all cases of body cavity fluids cases except in one case, UF-PAP stain gave a fewer score in the assessment of body cavity fluid samples.
Conclusion:
The findings of this study support the use of UF-PAP method in cytology laboratory with a high emphasis on FNA samples.
Keywords
Body cavity fluids
cytology
fine needle aspiration
papanicolaou
ultrafast papanicolaou
INTRODUCTION
In the last 20 years ago or so, changes in histopathology become evident in many ways. For example, the introduction of automated slide stainer in histology, autostainer in immunohistochemistry and SurePath® (TriPath Imaging, Inc., Burlington, NC, USA) and ThinPrep® 5000 (Cytyc Corp, Marlborough, MA, USA) systems in cytology. Among those, the modified ultrafast papanicolaou (UF-PAP) stain has been introduced. Originally, it was introduced to save reagents, lower the staining time, and possibly enhance the quality of staining.[123] The reception of this method in the developed countries was not great as the cost, staining time, and the quality of staining were not a major concern. However, in the developing countries, where the cost is a major issue, UF-PAP method could be a possible alternative with minimum compromise.
Although PAP stain is an excellent method to review the cytological specimens,[4] however, it is relatively time consuming, costly, and the detachment of materials from slides is another concern. Worldwide, several cytologists and cytopathologists tried different ways to modify PAP stain taking into their consideration the abovementioned limitations of PAP method. UF-PAP, rapid PAP, and rapid economic acetic acid PAP stain have been introduced in 1995, 2004, and 2008, respectively.[567] UF-PAP method received more acceptances, but it is still not in use in many countries.
According to our knowledge, most cytology laboratories in all Gulf countries including Oman, use the standard PAP method to stain various cytological specimens. Therefore, the aim of this study was to investigate the possible application of UF-PAP method in cytology laboratory, Sultanate of Oman.
MATERIALS AND METHODS
There were a total of 54 samples received in cytology laboratory at Sultan Qaboos University Hospital, Oman. Eight samples were excluded because they were unsatisfactory for diagnosis. The remaining 46 samples include nine thyroid fine needle aspiration (FNA) samples were collected from the Radiology Department, 11 breast FNA samples from breast clinic and 26 body fluid samples (containing 18 peritoneal, 6 pleural and 2 pericardial). This study was ethically approved by the Medical Research Committee and Ethics Committee (MREC #666) from the College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman.
Optimization
Several experiments were performed to optimize the ideal time for each staining step. For both stains, Harris hematoxylin and EA-50, we started with 10, 15, 20, 25, 30, 35, and 40 s. For Harris hematoxylin, it was found that 20 s gave good nuclei staining whereas for EA-50, 45 s gave good cytoplasmic staining. The main differences from the original method are shown in Table 1.[8]

Slides preparation
Body cavity fluids
Body cavity fluid samples were centrifuged for 5 min at 1800 rpm. Then, the supernatant was discarded from each sample. Three drops from the sediment of each sample were cytospinned for 5 min at 800 rpm. The slides were then removed from the cytospin. Two slides were immersed in 95% ethanol for subsequent staining with standard PAP stain. Other two slides were allowed to dry in the safety cabinet for staining with UF-PAP stain.
Fine needle aspiration
The slides were prepared by the clinicians immediately after FNA procedure using crash and pull technique. Two slides were immersed in 95% ethanol for staining with standard PAP stain. Other two slides were placed in the bottle without any fixative for staining with UF-PAP stain.
Staining methods
Standard papanicolaou stain
The slides were fixed in 95% ethanol for 15 min followed by immediate dipping in 50% ethanol for 2 min. After that, the slides were washed in tap water for 10 s. After the water had been removed from the slides using tissue papers, the slides were kept in Harris hematoxylin stain for 1 min. Then the slides were washed in tap water until clear. 0.5% acid alcohol was used for the differentiation of 2–3 quick dips. The nuclear stain was checked under the light microscope to ensure the clarity of the nuclei. The slides were washed in water for ten dips followed by ten dips in two changes of 95% ethanol. Immediately, the slides were placed in O-G-6 for 3 min. The slides were dipped in two changes of 95% ethanol for ten dips each. After that, the slides were placed in EA-50 for 4 min. The slides were dipped in three changes of 95% ethanol for ten dips each. Then, the slides were dipped in three changes of absolute ethanol for ten dips each. The slides were dipped in three changes of xylene for 15 dips each. Finally, the slides were mounted in DPX.
Ultrafast papanicolaou stain
Within half an hour of drying, the slides were placed in normal saline for 30 s to hydrolyze the blood and rehydrate the cells for good transparency. The slides were fixed in alcoholic formalin for 10 s to maintain the cell morphology in a live manner. The slides were washed in tap water for six dips. The slides were placed in Harris hematoxylin for 20 s. Then, the slides were washed in running tap water to remove the excess hematoxylin color. The slides were placed in 95% alcohol for six dips. The slides were placed in EA-50 for 45 s. The slides were washed in 95% alcohol for six dips. After that, the slides were washed in absolute alcohol for six dips. Finally, the slides were immersed in xylene for ten dips and mounted in DPX.
Screening and assessment
Background, nuclear staining, cell morphology, and overall staining were used as parameters to assess the quality of both staining methods.[8]
Background: If the slide was fully contaminated with red blood cells (RBCs), score 1 was given. When the slide contained a moderate number of RBCs, score 2 was given. If the slide was free of RBCs, score 3 was given. In thyroid slides, colloid also interferes with clarity of the background, so it was assessed in the same manner as with RBCs.
Cell morphology – Used to describe the cell shape. If the cells were well preserved and lacked any degenerative changes, they were given a score of 3. If cells were degraded, they were given score 1. Nuclear characteristics – Used to assess the clarity of nuclear details. If the details were not clear at all, score 1 was given. If the details were fully clear, score 3 was given. Overall staining – Used to assess the staining clarity, its distribution, and darkness or faintness. All the slides were reviewed independently by two cytoscreeners.
Statistical analysis
The results were analyzed using SPSS version 19 (Chicago, IL, USA). Corrected Chi-square test was used to determine the significance of the difference in percentages between the two staining methods for each parameter: Background nuclear staining, cell morphology, and overall staining. Differences in the statistical analysis of data were considered significant at P < 0.05.
RESULTS
Body cavity fluid
The clean background was seen in 38.5% of body cavity fluid samples using UF-PAP method whereas standard PAP showed only 11.5% of cases to be hemorrhagic. Nuclear details and overall staining were better seen with the standard PAP method as UF-PAP method showed less crisp nuclear details and overall staining was moderate. Also, morphological details of body cavity fluid samples were well preserved with standard PAP rather than UF-PAP method [Table 2].

Thyroid fine needle aspiration
The clean background was seen in 44.4% of thyroid FNA samples using UF-PAP method compared with only 11.1% with the standard PAP method [Figure 1]. Although the difference between the staining methods was not significant, cell morphology, nuclear details, and overall staining were better examined using the standard PAP method rather than UF-PAP method [Table 3].
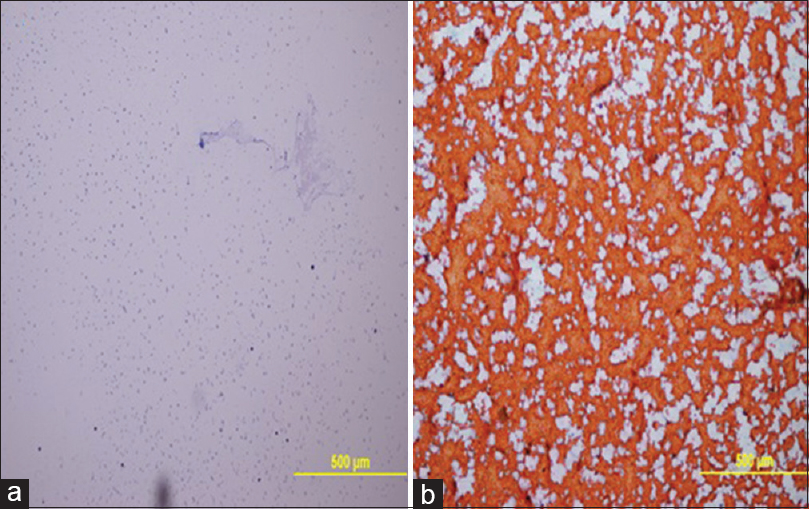
- Thyroid FNA. Clean background was observed in UF-PAP stain (a) whereas hemorrhagic background was observed in the standard PAP stain (b). (×100)

Breast fine needle aspiration
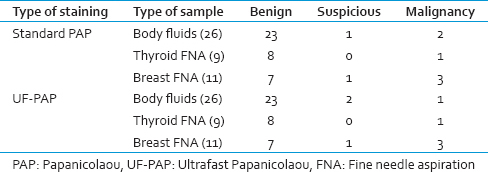
The clean background was similar with both staining methods. However, well preserved cell morphology, crisp nuclear details, and good overall staining were well seen with UF-PAP method when compared with the standard PAP method [Table 4]. The cytological diagnosis was similar using both staining methods except one case where it was reported as suspicious malignant with UF-PAP method and turned to be malignant with the standard method [Table 5].
DISCUSSION
In cytology, good screening makes the diagnosis accurate with minimum mistakes. Nuclear details, background, cell morphology, and overall staining are essential features for a successful screening.
In general, it was observed that the background was better in UF-PAP stain than in the standard PAP stain. The rehydration of air-dried smears in saline caused lysing of the RBCs. A better interpretation is possible if the epithelial cells were not obscured by RBCs. Only completely air-dried smears gave a clean background. Also, the delay in rehydration of air-dried smears or short rehydration time can lead to the incompletion of RBCs lysis and can give a dirty background. The rehydration time was set for 30 s as a standard. The smears should be rehydrated for enough time as soon as they are completely air-dried. The precaution should be taken that rehydration time should not be for a very long as it might affect the cytoplasmic details and dissolve noncellular components.[9] Another study found that the rehydration of air-dried smears was a suitable alternative to wet fixed smears.[8]
In breast FNA samples, UF-PAP and standard PAP methods gave the same score for background. There was no completely hemorrhagic background. Only moderate hemorrhagic background was observed in some samples and this could be due to the vascular nature of the lump. Little amount of blood cells in smears cannot affect the accuracy of the diagnosis.
Cell morphology of body cavity fluids in standard PAP was better than in UF-PAP stain. Although the difference in the percentage of cell morphology between the two staining methods was not significant. However, the poor quality of staining can lead to the wrong diagnosis. 3.8% of body cavity fluid samples showed poor preservation (degradation) of the cells. This could be due to the prolonged storage in the alcoholic formalin fixative. The pH of alcoholic formalin should be maintained at 5.0 and any change in pH of the solution can lead to poor preservation.[10] Also, insufficient fixation results in poor preservation.
Nuclear characteristics of body cavity fluids in the standard PAP were better than in UF-PAP stain and the difference in percentages was significant. The finding of this study disagreed with other study,[11] which concluded that UF-PAP improves the resolution of cytoplasmic and nuclear details of nonhematopoietic cells of body cavity fluids. In UF-PAP, the chromatin and nucleoli could not be distinguished in some cases. Also, the nucleus appeared as a very dark dot. In fact, prolonged storage, lower pH of the fixative, and rehydration time can affect the clarity of the nuclear details. A study showed that if the smear glass was left in alcoholic formalin for longer than 30 s, some of the nuclei were wrinkled and appeared blurred which might affect the accuracy of diagnosis.[12] Although, the correct diagnosis was made in all cases of body fluids samples except in one case that was reported as suspicious malignancy in UF-PAP stain, it was malignant in the standard PAP stain. This could be due to poor staining caused by the reasons discussed previously.
In thyroid FNA samples, there was no significant difference in percentages of the quality of staining, cell morphology, and nuclear characteristics in both staining methods. Although the UF-PAP stain showed 11.1% bad overall staining and 11.1% smudgy nucleus, these small percentages could be due to the technical errors such as slide preparation (crashing of the cells), pH maintenance, and the late dehydration. In most cases of thyroid FNA, UF-PAP stain smears were more cellular than standard PAP stain and this was due to the processing time of each technique. The finding of this study is inlined with another study that concluded that UF-PAP stain is one of the options to increase the sensitivity of follicular detection variant of papillary thyroid carcinoma in thyroid FNA.[13141516] Another study concluded that the diagnosis was possible in all cases of thyroid FNA cases using UF-PAP stain.[8]
In breast FNA samples, quality of staining, cell morphology, and nuclear characteristics were better in UF-PAP stain than in the standard PAP stain although the differences in percentage were not significant. The staining quality was excellent. The cell morphology was well preserved. The nuclei appeared large, open, and clear. The chromatin was crisp. Also, the diagnosis was possible in all cases of breast FNA cases. This finding is inlined with other studies.[91718]
To avoid poor staining due to weak solutions, it is very important to change the set of staining such as saline, alcoholic formalin, Harris hematoxylin, EA-50, and ethanol after a period. In this study, the fixative and saline were directly applied on each slide. Thus, the quality of fixative and saline was maintained for each slide and the contamination by other smeared materials was avoided. Also, Harris hematoxylin and EA-50 solutions were filtered after each ten slides and changed after each 20 slides. The metallic sheet in Harris hematoxylin was removed before the staining of each slide to avoid problems in the slides.
In addition to rapid diagnosis, UF-PAP stain has other advantages. Firstly, it can be used immediately to assess the material adequacy and whenever the stained material shows inadequacy, the aspiration can be repeated before releasing the patient. Secondly, immediate cytology enables more precise sample collection that leads to improved diagnostic accuracy while also improving the technical skill of the clinicians collecting the samples through immediate feedback.[19] Thirdly, the differences that existed in the stains of nuclear, chromatin, and cytoplasmic forms between these methods did not prevent the cytologist from reading any specimens after understanding the characteristics of each stain.[9] Fourthly, it is a very useful option for developing countries that suffer from a shortage of reagents.[6] Finally, UF-PAP procedure uses less alcohol and xylene changes with the omission of O-G-6 components when compared with the standard PAP method. In fact, another study reported that the cytomorphology processed by UF-PAP method is better than the quality of specimens prepared by ThinPrep method.[5]
As a limitation of this study, we should mention that UF-PAP stain is very sensitive technique, thus air-drying is a critical step. Also, due to the omission of O-G-6 in UF-PAP method, this method cannot be used for the diagnosis of squamous cell carcinoma. Therefore, urine and sputum samples were not included in this study. This may contribute to the small number of specimens presented in this study. Also, the reagents should be changed regularly. Furthermore, alcoholic formalin is very sensitive to pH changes, so optimal storage should be maintained.
CONCLUSION
The findings of this study support the use of UF-PAP method in cytology laboratory with a high emphasis on FNA samples.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank all the staff of Pathology Department at Sultan Qaboos University Hospital, Muscat, Sultanate of Oman, for their cooperation and helps in providing the date.
REFERENCES
- Modified Papanicolaou staining protocol with minimum alcohol use: A cost-cutting measure for resource-limited settings. Cytopathology. 2010;21:229-33.
- [Google Scholar]
- Papanicolaou stain: Is it economical to switch to rapid, economical, acetic acid, Papanicolaou stain? Acta Cytol. 2006;50:643-6.
- [Google Scholar]
- Ultrafast Papanicolaou stain: One year's experience in a fine needle aspiration service. Acta Cytol. 1997;41:1630-1.
- [Google Scholar]
- Report on a workshop of the UICC project on evaluation of screening for cancer. Int J Cancer. 1990;46:761-9.
- [Google Scholar]
- Ultrafast Papanicolaou stain. An alternative preparation for fine needle aspiration cytology. Acta Cytol. 1995;39:55-60.
- [Google Scholar]
- Rapid economic, acetic acid, Papanicolaou stain (REAP) – Is it suitable alternative to standard PAP stain? Al Ameen J Med Sci. 2008;1:99-103.
- [Google Scholar]
- Comparison of modified ultrafast Papanicolaou stain with the standard rapid Papanicolaou stain in cytology of various organs. J Cytol. 2012;29:241-5.
- [Google Scholar]
- Application of modified Ultrafast Papanicolaou stain in cytology of various organs. Diagn Cytopathol. 2006;34:135-9.
- [Google Scholar]
- Ultrafast Papanicolaou stain modified for developing countries: Efficacy and pitfalls. Acta Cytol. 2011;55:205-12.
- [Google Scholar]
- Application of Ultrafast Papanicolaou stain to body fluid cytology. Acta Cytol. 2001;45:180-5.
- [Google Scholar]
- Quick aspiration cytology for thyroid nodules by modified Ultrafast Papanicolaou staining. Diagn Cytopathol. 2003;28:45-8.
- [Google Scholar]
- Clear nuclei are specific to papillary carcinoma in thyroid fine-needle aspirates processed by Ultrafast Papanicolaou stain. Diagn Cytopathol. 2003;29:236-7.
- [Google Scholar]
- Ultrasound-guided fine-needle aspiration of the thyroid assessed by Ultrafast Papanicolaou stain: Data from 1135 biopsies with a two- to six-year follow-up. Thyroid. 2001;11:581-9.
- [Google Scholar]
- Efficacy of a modified Ultrafast Papanicolaou (UFP) stain for breast aspirates. J Indian Med Assoc. 2004;102:309, 312, 326.
- [Google Scholar]
- Diagnostic accuracy of follicular variant of papillary thyroid carcinoma in fine-needle aspirates processed by Ultrafast Papanicolaou stain: Histologic follow-up of 125 cases. Cancer. 2006;108:174-9.
- [Google Scholar]
- Effect of Ultrafast Papanicolaou staining on nuclear and textural features in breast cancer cytology. Anal Quant Cytol Histol. 1997;19:361-7.
- [Google Scholar]
- Efficacy of a modified Ultra Fast Papanicolaou (UFP) stain for breast aspirates. Indian J Pathol Microbiol. 2000;43:417-21.
- [Google Scholar]
- Utility of immediate cytologic diagnosis of lung masses using Ultrafast Papanicolaou stain. Lung Cancer. 2011;72:172-6.
- [Google Scholar]





