Translate this page into:
Cutaneous Clear Cell Sarcoma: A Rare Aggressive Tumor with Potential Diagnostic Challenge
Address for correspondence: Dr. Akshay Bali, E-mail: aks23bali@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Clear cell sarcoma is a deep-seated, exceedingly rare aggressive tumor, typically involving the tendons and aponeuroses with melanocytic differentiation and a distinct genetic background. A primary dermal location is rarer. It exhibits histological, immunohistochemical, and ultrastructural similarities with the more common primary (or metastatic) malignant melanoma causing major diagnostic confusion. We describe a case of primary cutaneous clear cell sarcoma arising in the right lower extremity of a 40-year-old male patient.
Keywords
Clear cell sarcoma
cutaneous
malignant melanoma
INTRODUCTION
Clear cell sarcoma (CCS) is an exceedingly rare tumor of young adults with melanocytic differentiation.[12] The exact incidence is largely unknown, although occasional case series mention CCS comprising less than 1% of all soft tissue sarcoma.[3]
CCS is a deep-seated tumor, typically involving tendons and aponeuroses. It has a predilection for lower extremities, particularly around the foot and ankle region, accounting for nearly 40% of cases.[13] A primary dermal origin is rarer.
The histological picture is dominated by nests of uniform polygonal to fusiform eosinophilic or clear cells with vesicular nuclei and prominent nucleoli delineated by fibrous septa. The immunohistochemical and ultrastructural evidence of definite melanocytic differentiation led it to be also designated malignant melanoma of soft parts.[14] A characteristic cytogenetic abnormality t (12;22) (q13;q12) can be detected in 70-90% cases of CCS.[4–6]
The histological similarity and the immunohistochemical overlap pose a protean challenge in diagnosing and distinguishing cutaneous CCS from the more common primary (or metastatic) malignant melanoma (MM).[47]
We report a case of primary cutaneous CCS located on the right leg of a 40-year-old male patient.
CASE REPORT
A 40-year-old male patient presented with a painful nodule in the lower part of right leg. He related that the nodule had remained stable in size and non-painful for 4 years. Over the past 6 months, it began to enlarge and became painful. There was no history of trauma, and his personal or family history was negative for any malignancy. On palpation, an irregular, firm, erythematous, mobile, tender mass measuring 3.5 × 2.3 × 1.7 cm, was noted just above the lateral malleolus of the right leg. Popliteal and inguinal lymph nodes were not palpable. A thorough clinical examination did not reveal other relevant cutaneous lesions.
Systemic examination and investigations including complete blood count, fasting blood sugar level, liver function test, and renal function test were within normal limits. Hepatitis B virus surface antigen and human immunodeficiency virus enzyme-linked immunosorbent assay were non-reactive.
The patient underwent local excision of the tumor. A curvilinear approach was employed for maximum exposure of the tumor, which was removed completely and submitted for histopathological examination.
Gross inspection revealed a lobulated, tan-grey, firm mass, measuring 3.5 × 2.3 × 1.7 cm, and partly covered with skin. Light microscopy revealed a poorly-delineated dermal neoplasm, extending into the underlying superficial parts of the subcutis. The tumor was composed of nests and fascicles of spindle-shaped to epithelioid cells, with pale eosinophilic cytoplasm and vesicular nuclei with prominent nucleoli infiltrating a collagenous stroma. Mitoses, including atypical, amounting to 7-8 mitotic figures per 10 high power-fields were noted. Intracytoplasmic melanin pigment was not noted. The epidermis did not show any significant increase in melanocytes within the basal layer, junctional activity or pagetoid spread of tumor cells [Figures 1 and 2].
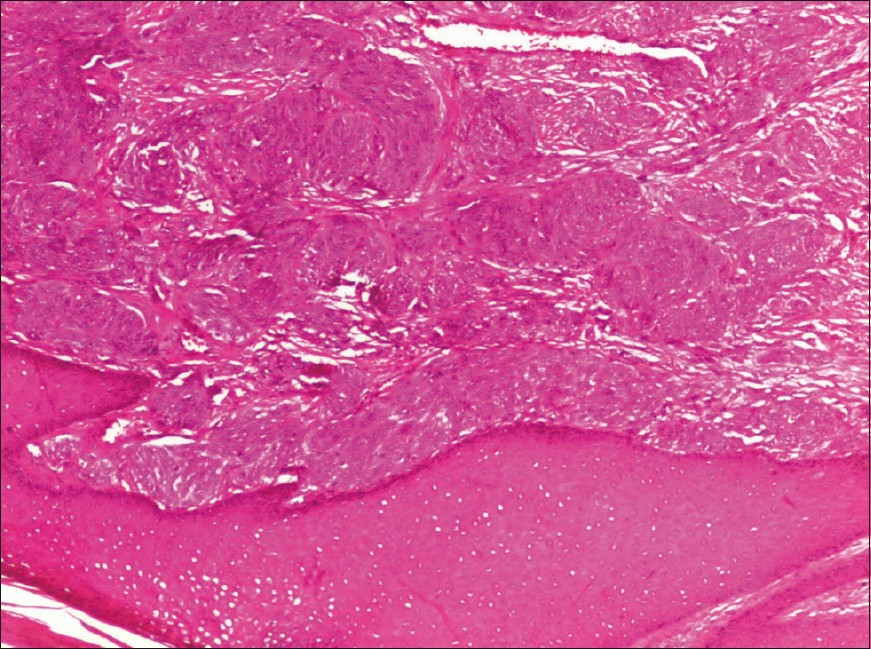
- Clear cell sarcoma: low power revealed a dermal malignancy composed of tumor cell nests and fascicles delineated by fibrous septa. No junctional activity or pagetoid spread of abnormal melanocytes (H and E, ×100)
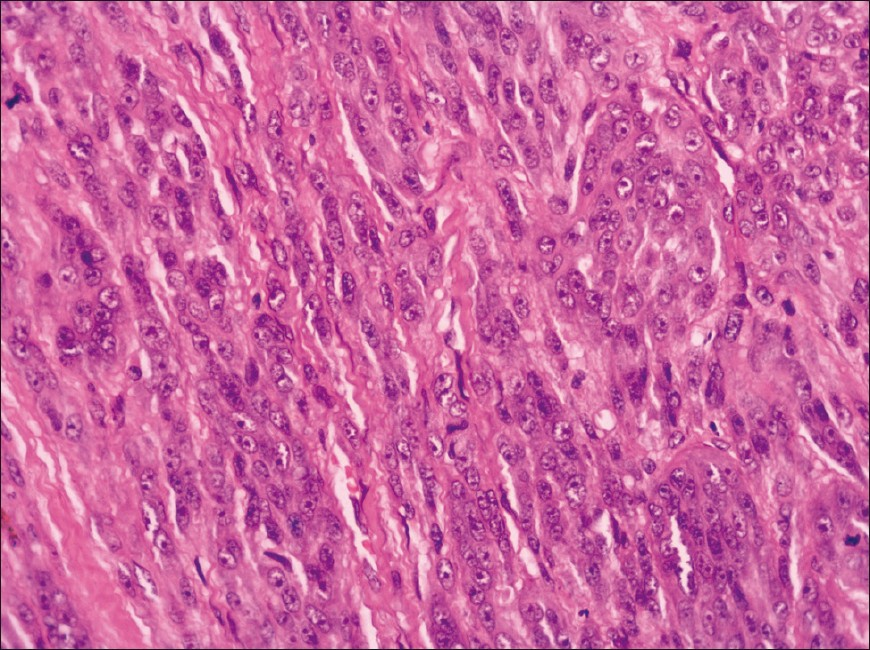
- Clear cell sarcoma: Higher magnification showing spindled to epithelioid cells with pale eosinophilic cytoplasm and vesicular nuclei with prominent nucleoli (H and E, ×400)
On immunohistochemistry, the tumor cells exhibited strong positivity for vimentin and focal positivity for Melan A and HMB-45. The neoplastic cells stained negative for S-100 protein, pancytokeratin, epithelial membrane antigen, and CD 31 [Figure 3]. A diagnostic dilemma occurred between MM and cutaneous CCS. However, in view of lack of epidermal changes and absence of any identifiable primary cutaneous neoplasm, a final diagnosis of cutaneous CCS was rendered.
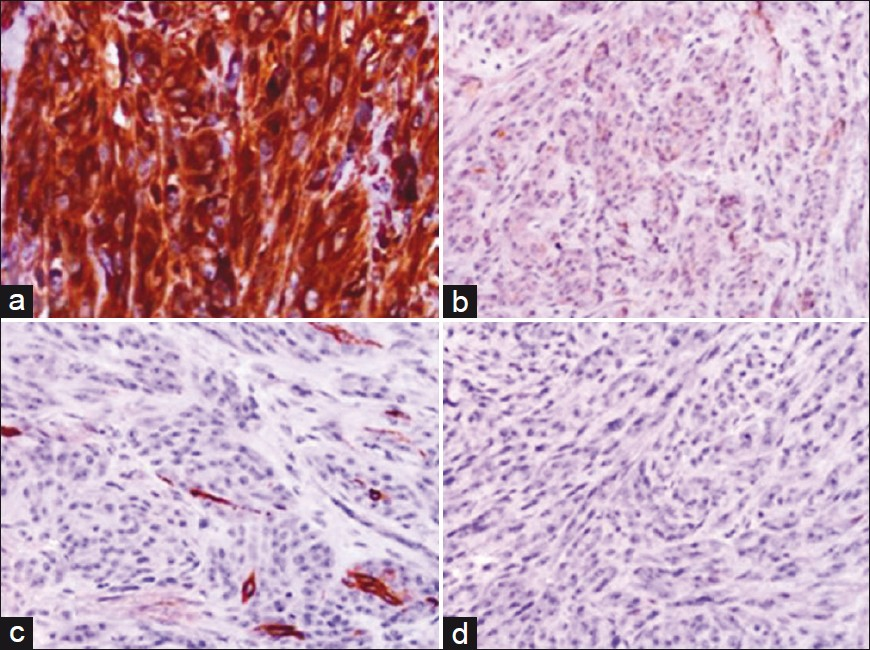
- Immunohistochemical staining showing (a), Intense cytoplasmic immunopositivity for vimentin (Vimentin ×400); (b), Cytoplasmic immunoreactivity for Melan-A (Melan-A × 400); (c): Focal cytoplasmic immunoreactivity for HMB-45 (HMB-45 ×400); (d), Immunonegative for S-100 protein (S-100, ×400)
The patient declined to undergo revision of the initial surgery with a wider margin of resection and sentinel lymph node biopsy and discharged himself against medical advice.
DISCUSSION
Dr. Franz Enzinger is credited with the first description of this unique sarcoma in 1965 and coined the term CCS of tendons and aponeuroses.[8] In his subsequent paper coauthored with Chung in 1983, the author duo documented the melanocytic phenotype of the tumor and proposed the term malignant melanoma of soft parts over the purely descriptive term of CCS.[9]
Adolescents and young adults comprise the most common age group affected with no particular gender bias.[18] It commonly occurs in the deep soft tissues, juxtaposed to tendons, fascia or aponeuroses and mostly present as a slow-growing and frequently painful (30-60%) soft tissue mass. Although foot and ankle are the most frequently involved sites, rare cases presenting in the kidney, trunk, penis, gastrointestinal tract, head, and neck have been reported.[1] A primary dermal location is extremely rare with few isolated case reports and only series of 12 cases by Hantschke et al,[4] reported till date.
Most tumors are relatively small, ranging from 0.4 cm up to 14.5 cm in greatest dimension.[1] Histologically, CCS classically displays compact nests and fascicles of uniform to minimally pleomorphic tumor cells delineated by dense fibrous septa. The neoplastic cells are polygonal or spindle-shaped with abundant clear or pale eosinophilic cytoplasm and a centrally-located round to ovoid vesicular nuclei with prominent nucleoli. Mitotic activity is generally low, and scattered wreath-like multinucleated giant cells are encountered in 50% of cases.[145] The tumor cells are immunopositive for the common melanocytic markers, namely HMB-45, microphthalmia transcription factor (MITF), S-100 protein, and Melan-A in 90%, 71%, 64% and 43% cases, respectively. Ultrastructurally melanosomes are usually detected.[1] A reciprocal translocation t (12;22) (q13;q12) resulting in a EWSR1/ATF1 chimeric transcript, identifiable in 70-90% cases, is considered the cytogenetic hallmark of CCS.[1–6]
MM, primary or metastatic, with its histological, immunohistochemical and ultrastructural overlap constitutes the most important diagnostic mimic of cutaneous CCS.[14] However, Hantschke et al,[4] outlined several reliable histologic criteria for the accurate distinction between CCS and MM. CCS is most often characterized by hyalinized sclerotic and reticulated stroma with fascicles of uniform population of tumor cells encased by delicate fibrous septa, a pattern that is seldom observed in MM. Moreover, CCS does not display any pagetoid spread of atypical melanocytes and commonly features tumor giant cells with characteristic wreath of multiple peripherally-placed nuclei. Ultimately, the t (12;22) (q13;q12) translocation observed in most cases of CCS has not yet been identified in MM.[14] The other differential diagnosis of CCS located in the extremity include paraganglioma-like dermal melanocytic tumor, clear cell myelomonocytic tumor, malignant peripheral nerve sheath tumor, and synovial sarcoma, especially the monophasic type. A careful histological evaluation coupled with immunohistochemical demonstration of melanocytic differentiation in CCS usually establishes the diagnosis.[1]
CCS is an aggressive tumor with propensity for recurrences, early metastases and therefore, poor overall survival.[13] Adverse prognostic factors include tumor size more than 5 cm, and presence of microscopic tumor necrosis.[14] Surgery, involving a wide excision of the tumor with sentinel lymph node biopsy, constitutes the mainstay of treatment with chemotherapy and radiotherapy showing no proven beneficial effect.[12]
In conclusion, cutaneous CCS is a rare unique aggressive tumor with histological and immunohistochemical similarities to MM but harbors a distinct genetic background.[145]
ACKNOWLEDGEMENTS
To the department of surgery, KLESH Dr. Prabhakar Kore Hospital and Medical Research Center for providing us with a interesting case material, and Dr. Sanjay Navani for his valuable help in performing immunohistochemistry.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Clear cell sarcoma of tendons and aponeuroses: A review. Arch Pathol Lab Med. 2007;131:152-6.
- [Google Scholar]
- Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathological study of 30 cases. Cancer. 1999;86:969-75.
- [Google Scholar]
- Clear cell sarcoma of the scapula. A case report and review of literature. World J Surg Oncol. 2006;4:48-53.
- [Google Scholar]
- Cutaneous clear cell sarcoma: A clinicopathological, immunohistochemical, and molecular analysis of 12 cases emphasizing its distinction form dermal melanoma. Am J Surg Pathol. 2010;34:216-22.
- [Google Scholar]
- Clear cell sarcoma of soft tissue: A clinicopathological, immunohistochemical, and molecular analysis of 33 cases. Am J Surg Pathol. 2008;32:452-60.
- [Google Scholar]
- Dual-color, break-apart fluorescence in situ hybridization for EWS gene rearrangement distinguishes clear cell sarcoma of soft tissue from malignant melanoma. Mod Pathol. 2005;18:1585-90.
- [Google Scholar]
- Clear cell sarcoma: A case mimicking primary cutaneous malignant melanoma. Indian J Dermatol. 2009;54:168-72.
- [Google Scholar]
- Clear cell sarcoma of tendons and aponeuroses: An analysis of 21 cases. Cancer. 1965;18:1163-74.
- [Google Scholar]
- Malignant melanoma of soft parts.A reassessment of clear cell sarcoma. Am J Surg Pathol. 1983;7:405-13.
- [Google Scholar]





