Translate this page into:
Diagnostic utility of quantitative cytomegalovirus DNA polymerase chain reaction in intestinal biopsies from patients with inflammatory bowel disease
Address for correspondence: Dr. Ekta Gupta, Department of Clinical Virology, Institute of Liver and Biliary Sciences, Vasant Kunj, New Delhi - 110 070, India. E-mail: ektagaurisha@gmail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
OBJECTIVES:
Diagnostic utility of cytomegalovirus (CMV) DNA quantitative polymerase chain reaction (qPCR) in inflammatory bowel disease (IBD) has not been established. We aimed to compare diagnostic utility of qPCR for CMV in biopsy specimens with blood, serology, and histopathology.
MATERIALS AND METHODS:
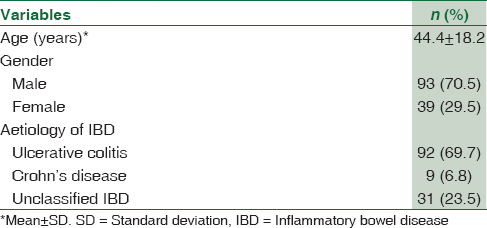
A total of 132 patients were included (92 ulcerative colitis [UC], 9 Crohn's disease, and 31 unclassified IBD). Comparison between CMV IgM, CMV DNA qPCR in biopsy, in blood and histopathology was done. Positive result in any of the test was considered as CMV infection. Various risk factors for CMV association with IBD were analyzed.
RESULTS:
Confirmed CMV infection was seen in 41 (31.1%) patients. Diagnostic sensitivity of different assays was: DNA in biopsy seen in 37 (90.2%), DNA in blood in 19 (46.3%), CMV IgM in 15 (36.5%), and histopathology in 8 (19.5%). Thirty-two UC cases were further followed up for a median time of 14.0 (R: 3–31) months. They were grouped as group I – biopsy and blood DNA both positive (14, 43.7%), Group II – biopsy positive and blood negative (17, 53.1%), and Group III – biopsy negative but blood positive (1, 3.1%). CMV DNA viral load in Group I was significantly higher (mean: 4.2 ± 1.0 log10 copies/mg) than Group II (mean: 3.2 ± 0.6 copies/mg) and Group III (viral load: 2.69 log10 copies/ml), P < 0.001. Steroid refractoriness was seen more in Group I cases (n = 9) P < 0.001. A cutoff of ≥2.5 log10 copies/mg of DNA in tissue was predictive for steroid refractoriness (AUROC = 0.84).
CONCLUSIONS:
Quantitation of CMV DNA in intestinal biopsy is a useful diagnostic tool and can predict response to steroid treatment in patients with UC.
Keywords
Cytomegalovirus
inflammatory bowel disease
quantitative polymerase chain reaction
ulcerative colitis
Introduction
Cytomegalovirus (CMV) is a member of the Herpesviridae family and is a common infection in humans with a prevalence of over 70%. As with most of these viruses once the infection is acquired (primary infection), CMV remains in the host in a state of lifelong latency from which it can be reactivated.[1] The most CMV infections are acquired either in the perinatal period and infancy or in adulthood through sexual contact. CMV targets epithelial cells lining the respiratory or gastrointestinal tract in primary infection.[2] Primary infection in immunocompetent host runs a very mild asymptomatic course, but in immunocompromised (patients with AIDS, organ transplantation, cancer chemotherapy, steroids, or other immunosuppressive), CMV can cause severe disease affecting the gastrointestinal tract, the lung, the retina and the liver. In the gastrointestinal tract, CMV can cause colitis, esophagitis, gastritis, ulcers, terminal ileitis, intestinal perforation, and pouchitis.[1]
The first report of intestinal CMV infection in a patient with ulcerative colitis (UC) was published in 1961.[3] Since then, there has been ongoing controversy about the clinical relevance of CMV infection in patients with inflammatory bowel disease (IBD), especially severe, and steroid-refractory UC.[456] It remains a matter of debate whether CMV infection can lead to relapse in UC or alter its natural history. The presence of CMV has been reported in the colonic tissue of 21%–34% of patients with acute severe colitis and to 33%–36% of the steroid refractory subgroup of UC patients.[478] The available methods to diagnose intestinal CMV infection include histology, serology, polymerase chain reaction (PCR) for CMV DNA in the blood/intestinal biopsy, CMV pp65 antigen.[9] CMV DNA PCR from intestinal biopsies was shown to have the highest sensitivity above all methods to diagnose CMV infection in IBD patients.[9] Clinical guidelines suggest that in patients with refractory IBD flares, before escalation of immunosuppressive therapy, CMV diagnostics should be initiated, preferably by tissue DNA PCR or immunohistochemistry (IHC).[10]
Very few studies have measured CMV DNA load as a marker of disease progression in the intestinal tissue of IBD patients and to predict response to steroids.[611]
Therefore, in this study, we tried to evaluate the clinical usefulness of CMV DNA quantification in UC patients and correlate it with steroid refractory status.
Materials and Methods
Study population
This retrospective study was done in the department of virology from April 2011 to April 2016. All patients with clinical suspicion of IBD whose blood and intestinal biopsy were sent to the virology laboratory for CMV diagnosis were included in the study. Clinical details and follow-up data were obtained from the hospital information system. The present study was approved by the institute ethical board.
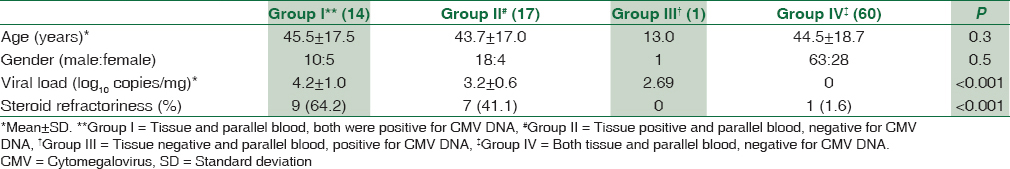
UC patients with CMV DNA positivity were further followed up for a median period of 14.0 (3–31) months, and they were grouped into three groups to compare various demographic and clinical parameters. The Group I consisted of those cases who were positive for CMV DNA, both in biopsy as well as blood samples. Group II was biopsy positive, but blood negative and Group III were biopsy negative and blood positive. The patients who were negative for CMV DNA (biopsy + blood) were grouped as Group IV.
Data on drugs used in treatment for IBD before the collection of sample for detection of CMV infection was noted. As per institutional treatment policy, UC patients were treated with Steroid (40 mg once a day with tapering of dose, 10 mg reduction/week) and azathioprine (100 mg once a day). The patients who were on steroids and did not show any clinical improvement despite being on constant dose for at least 2 weeks were considered as steroid refractory, as per the defined clinical criteria.[12]
In all the samples, CMV IgM, CMV DNA real-time quantitative PCR (qPCR) was done. Histopathological analysis of all biopsy samples was done by hematoxylin and eosin (H and E) staining, and CMV infection was defined as characteristic cytomegalic cells and “owl's eye” nuclear inclusion bodies. Immunohistochemistry IHC was performed for confirmation on samples that were positive for CMV infection on histopathology. CMV infection in patients was considered if any of the above tests was positive for CMV.
Once the patient is diagnosed with CMV infection, they were put on valganciclovir (450 mg twice a day) for 2 weeks. The patients were followed up for clearance of CMV DNA in blood.
Cytomegalovirus IgM serology
All blood samples were collected in ethylenediaminetetraacetic acid vials and plasma was separated and stored at 80 till further processing. Anti-CMV IgM antibody was tested in all serum samples by chemiluminescent microparticle immunoassay using a commercially available kit (Abbott, Architect, Ireland) using the positive and negative controls provided with the kit as per the manufactures instruction. The results given as signal/cutoff, and the cutoff values were calculated based on each individual run to determine each sample as reactive or nonreactive. An index value of >1 was considered as positive.
Cytomegalovirus DNA real-time polymerase chain reaction
Extraction of DNA from intestinal biopsy and blood
All the biopsy samples were collected in sterile plain vial and transported to laboratory on ice. Extraction of DNA from biopsy tissue sample was done using the automated DNA extraction system (QIAsymphony, Qiagen, Germany) using DSP virus/pathogen kit, with slight modifications. Tissue was weighed and 5 mg of the tissue was digested by proteinase K before processing. Volume was adjusted to 750 μl with AVE buffer (Qiagen, Germany). Plasma (750 μl) samples were directly processed in the automated Qiasymphony, as per manufacturer instructions.
Real-time polymerase chain reaction for cytomegalovirus DNA quantification
QPCR for CMV was performed in both biopsy and plasma samples in all the patients using the artus CMV RG PCR kit (Qiagen, Hilden, Germany), as per the manufacturer's instructions. The assay targets a 105 bp region of the glycoprotein gene of CMV genome. An internal control was added to each sample before extraction and each batch of PCR included four positive and one negative control. CMV viral load levels were then expressed as the number of CMV DNA copies/mg of tissue for intestinal biopsy sample and in copies/mL for plasma sample. The dynamic range of the assay is 102–106 copies/mL with a lower limit of detection of 57.1 copies/mL.
Statistical analysis
Categorical variables were presented as proportions while continuous variables were either presented as mean with standard deviation or median with range. Comparison of continuous variables was done by one-way ANOVA with Post hoc by Bonferroni method or Kruskal–Wallis test and categorical variables by Fisher's exact test or Pearson's Chi-square test. Receiver operating characteristic (ROC) curve was used to determine a cutoff point of categorical predictors. All statistical tools were two tailed, and a significant level (P < 0.05) was used. All statistical tests were performed using SPSS for Windows version 22 (IBM Corp, Armonk, USA).
Results
Out of 132 clinically, suspected IBD patients, a diagnosis of UC based on histopathology was made in 92 (69.7%), followed by CD in 9 (6.8%) patients as shown in Table 1.
Comparison of various diagnostic assays for cytomegalovirus infection
Overall CMV infection was seen in 41 (31.1%). Of the 41 CMV positive patients, detection by qPCR in biopsy was seen in 37 (90.2%); in 19 (46.3%) blood samples, CMV IgM serology was positive in 15 (11.4%) and histopathology in 8 (6%) cases. Diagnostic sensitivity of qPCR in the biopsy, qPCR in the blood, IgM antibody in the serum, and histological examination of CMV inclusion body in biopsy was 90.2%, 46.3%, 36.5%, and 19.5%, respectively.
Group-wise comparison of various risk factors and follow-up of the cytomegalovirus DNA-positive ulcerative colitis patients
Out of total 41 CMV DNA-positive cases, association with UC was seen in 32 patients followed by three in CD and six in unclassified IBD. Further follow-up of UC patients was done. All UC patients were managed by steroid, and in 11 patients, azathioprine (100 mg OD) was also added. UC patients with CMV infection were grouped as described earlier [Table 2]. Group I comprises 14 (43.7%) cases where CMV DNA qPCR was dually positive in tissue biopsy and parallel blood samples; Group II was those cases 17 (53.1%) who were only positive for CMV DNA qPCR in biopsy samples but not in parallel blood samples. Group III had only 1 (3.1%) case who was positive for CMV DNA qPCR in blood sample but not in biopsy tissue. CMV DNA viral load in Group I was significantly higher (mean: 4.2 ± 1.0 log10 copies/mg) than Group II (mean: 3.2 ± 0.6 copies/mg) and Group III (viral load: 2.69 log10 copies/ml), P < 0.001. Furthermore, patients under immunosuppressive agent (steroid) had a significant association with dual positivity (Group I) than other groups (P < 0.001).
When these patients were put on CMV-specific treatment (oral valganciclovir 450 mg twice a day for 2 weeks), majority of the patients showed improvement in terms of clearance of CMV DNA from the blood. Only in two patients of Group I a colectomy procedure was done and one patient in Group II died [Figure 1].

- Clinical outcome of the cytomegalovirus DNA-positive patients
Correlation of cytomegalovirus DNA viral load with steroid refractory status
When CMV DNA positivity was compared with steroid refractory status, a random sensitivity analysis was performed to correlate the presence of CMV DNA in tissue with the occurrence of refractoriness to steroid treatment. A positive colonic CMV viral load was associated with an increased risk of steroid resistance. AUROC curve for diagnosing refractoriness status was 0.84 (95% confidence interval [CI] = 0.73–0.94; P < 0.001). The cutoff value of CMV DNA viral load that could predict steroid refractoriness with a sensitivity of 79.2%, and a specificity of 84.3% was found to be ≥2.5 log10 copies/mg of tissue (P < 0.001) [Figure 2].

- Receiver operating characteristic curve showing relationship between intestinal cytomegalovirus viral load and response to immunosuppressive therapy
Discussion
In this study, CMV infection was seen in 31.1% of IBD cases by CMV DNA qPCR, CMV IgM serology in 11.4%, and histopathology in only 8 (6%) cases, thereby showing that qPCR is a better diagnostic tool for the identification of CMV as compared to serology and histopathology.
The prevalence of CMV in IBD patients is sparsely described ranging from 3% to 60%, depending on the diagnostic modality used by the authors.[13] Most of the studies are either on few selected groups of patients or using limited diagnostic modalities. Hence, the prevalence range varies widely. In the present study, the prevalence of CMV in IBD patients was in agreement with the existing literature and toward the higher side as the method used in the present study was CMV DNA detection in both the tissue biopsy and blood samples.
Out of the various diagnostic modalities used by different authors worldwide, the sensitivity of PCR in colonic biopsy has shown to be highest, i.e., around 60%–90%, whereas for CMV IgM serology and histopathology is around 15%–60% and 5%–19%, respectively.[91415] Although histopathology is considered as the gold standard for diagnosing CMV infection, it is observer dependent and the characteristic features of CMV infections such as inclusion bodies and cytomegalic cells are not always seen in routinely performed H and E stain.[16] Even by doing IHC, the sensitivity has not shown to be as good as CMV DNA PCR.[17] Real-time PCR further increases the sensitivity of the PCR assays as the lower limit of detection is as low as 50 copies; moreover, viral load quantification also allows us to monitor the treatment response of the patient.[615] In the present study, qPCR for CMV DNA detection as well as quantification was used and was also used to follow-up the patients on treatment. In this study, qPCR for CMV DNA in biopsy demonstrated better sensitivity than other modalities.
It has been reported that the risk of CMV reactivation differs in UC and CD because the relative amounts of tumor necrosis factor-alpha and interferon γ favors reactivation in UC more than in CD. This is supported by reports showing that CMV colitis is more common in UC.[2] Therefore, in the present study, the association of CMV DNA with UC cases was only studied. The patients were grouped based on tissue biopsy DNA positivity and blood positivity as it has been reported in literature that during active replication of the virus in IBD patients, viral particles are shed in blood as well as in the lumen of the gut, and the role of neither of the specimens has been established so far.[18] This study clearly highlights the utility of dual PCR for establishment of CMV infection, as viral load was much higher in CMV DNA dual-positive cases as compared to those who were only biopsy or blood positive. This may be because dual positivity as demonstrated by the presence of CMV DNA both in biopsy sample as well as blood indicates a true association of CMV with patients of UC. By contrast, the presence of CMV DNA in peripheral blood in the absence of CMV DNA in biopsy or confirmation in histopathology could have been due to reactivation of CMV from any other site apart from gut.[19]
In majority of the published studies, CMV is considered as a true pathogen, complicating the course of UC[220212223] while in few studies, the role of CMV has been debated in influencing the natural course of the underlying UC.[2425] In the present study, CMV was found to be associated with steroid refractory status of the patient and administration of CMV-specific treatment helped in clinical improvement thereby establishing the pathogenic role of CMV in UC patients.
The detection of CMV DNA in biopsy samples though confirms CMV infection, but there is no consensus on cutoff value of CMV DNA viral load that could help in predicting the treatment response. It is not clear that what should be the cutoff value of CMV DNA that would define CMV disease and initiation of CMV-specific treatment.[2627] Various studies have defined their own cutoff values for starting antiviral treatment.[1823] In our Institute, there is no defined CMV DNA load to start the CMV-specific treatment.
It has also been observed that UC patients with CMV infection were more prone to develop steroid refractoriness.[1726] and a cutoff value of CMV DNA to predict the steroid refractoriness is yet to be identified. There is one study by Roblin et al.,[6] have shown, a CMV DNA load above 250 copies/mg in the tissue was predictive of resistance to three successive immunosuppressive regimens (likelihood ratio + = 4.33; area under the ROC curve = 0.85). In their study, ganciclovir was given to eight UC patients with CMV DNA in inflamed tissue and therapeutic failure; 7 (87.5%) achieved clinical remission. Whereas, in the current study, we have found that a CMV DNA load of 2.5 log10 copies/mg of tissue was predictive of steroid refractoriness and when they were put on CMV-specific antiviral therapy, majority of the patients responded well.
It has been reported in various studies that the incidence of steroid refractoriness ranges from 16% to 30% in patients with UC and 16%–20% and in patients with CD.[14152021] The mechanism that causes steroid refractoriness is still unknown. In many previous studies, it was suspected that steroid refractoriness during IBD was secondary to CMV infection. The latest meta-analysis also reported significantly higher steroid refractoriness (70%) in CMV-positive cases than in CMV-negative cases (relative risk = 2.12, 95% CI = 1.72–2.61).[20] In our study also, steroid refractory status of UC patients with CMV infection was 50.0% which was in concordance with the result of previous studies.[671415202122]
CMV infection is commonly seen in patients with severe ulcerative colitis. It is likely that the infection is mediated by the both inflammation of the mucosa and the immunosuppressive drugs given to such patients. In UC Patients with CMV infection likely to have poor outcome than those patients without infection as clearly evident in the current study. However, the treatment of CMV infection in patients with severe colitis might reduce the colectomy rate suggesting that the virus is playing a role in the poor outcome of patients with severe UC. Therefore, an early detection and quantitation of CMV DNA in intestinal biopsy is of utmost importance for the correct management of UC patients with CMV infection patients.
Conclusions
This retrospective study demonstrates that dual CMV DNA qPCR (biopsy + plasma) is a better diagnostic method than histopathology and CMV IgM serology. Overall, our results suggest that CMV infection at the tissue level as well as in the blood is not an innocent bystander in the course of UC but plays a key role in response to immunosuppressive treatments. Thus, an early detection of CMV infection as well as quantitation by qPCR will help in the correct management of such patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Vijeta Bajpai, Senior resident, Virology, ILBS and Mr. Nadeem Hasnain, Senior research fellow, Virology, ILBS.
References
- Cytomegalovirus and inflammatory bowel disease: Pathogenicity, diagnosis and treatment. Ann Gastroenterol. 2007;20:110-5.
- [Google Scholar]
- Review article: Cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:725-33.
- [Google Scholar]
- Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30:334-40.
- [Google Scholar]
- Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006;101:2857-65.
- [Google Scholar]
- Management of cytomegalovirus infection in inflammatory bowel diseases. Dig Liver Dis. 2012;44:541-8.
- [Google Scholar]
- Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;106:2001-8.
- [Google Scholar]
- Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am J Gastroenterol. 2001;96:773-5.
- [Google Scholar]
- Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J Gastroenterol. 2016;22:2030-45.
- [Google Scholar]
- Infection with cytomegalovirus in patients with inflammatory bowel disease: Prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155-60.
- [Google Scholar]
- Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-68.
- [Google Scholar]
- Cytomegalovirus infection in patients with ulcerative colitis diagnosed by quantitative real-time PCR analysis. Dig Dis Sci. 2006;51:1052-5.
- [Google Scholar]
- Clinicopathologic characteristics of clinically relevant cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2007;42:823-9.
- [Google Scholar]
- Clinical significance of cytomegalovirus infection in patients with inflammatory bowel disease. World J Gastroenterol. 2013;19:17-25.
- [Google Scholar]
- The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: A prospective multicenter study. J Clin Gastroenterol. 2012;46:51-6.
- [Google Scholar]
- Steroid-refractory inflammatory bowel disease is a risk factor for CMV infection. Eur Rev Med Pharmacol Sci. 2016;20:858-65.
- [Google Scholar]
- Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:423-9.
- [Google Scholar]
- Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007;13:1516-21.
- [Google Scholar]
- The pathogenicity of cytomegalovirus in inflammatory bowel disease: A systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis. 2004;10:245-50.
- [Google Scholar]
- Cytomegalovirus is frequently reactivated and disappears without antiviral agents in ulcerative colitis patients. Am J Gastroenterol. 2007;102:331-7.
- [Google Scholar]
- Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J Gastroenterol. 2011;17:633-8.
- [Google Scholar]
- Cytomegalovirus infection of the colon: A possible role in exacerbations of inflammatory bowel disease. Am J Gastroenterol. 1985;80:355-60.
- [Google Scholar]
- Cytomegalovirus enteritis: A highly lethal condition requiring early detection and intervention. Dis Colon Rectum. 1998;41:619-23.
- [Google Scholar]
- Ulcerative colitis exacerbation associated with cytomegalovirus infection. Hong Kong Med J. 1998;4:437-39.
- [Google Scholar]
- Severe acute colitis associated with CMV: A prevalence study. Dig Liver Dis. 2004;36:818-20.
- [Google Scholar]
- Prevalence, detection rate and outcome of cytomegalovirus infection in ulcerative colitis patients requiring colonic resection. Dig Liver Dis. 2005;37:418-23.
- [Google Scholar]
- Intractable ulcerative colitis caused by cytomegalovirus infection: A prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46:S59-65.
- [Google Scholar]
- Cytomegalovirus infection in ulcerative colitis: A prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008;14:1373-9.
- [Google Scholar]





