Translate this page into:
Efficiency Assessment of Immunochromatographic Strip Test for the Diagnosis of Alpha-Thalassemia-1 Carriers
Address for correspondence: Dr. Yuttana Sudjaroen, E-mail: yuttana.su@ssru.ac.th
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
The prevention and control of thalassemia in Thailand focus on the appropriate diagnosis with a simple, cheap, and practical tool for any staff to use.
Aims:
(1) To screen alpha-thalassemia-1 carriers among pregnant women and spouses by immunochromatographic (IC) strip test and one tube osmotic fragility test (OFT) and (2) to evaluate the accuracy of both screening methods.
Setting and Design:
Cross-sectional study for 6 months duration from January to June 2013 at Kudjab Hospital located in Udonthani Province, Thailand.
Subjects and Methods:
Pregnant women and spouses attending the antenatal care clinic joined the study for blood sample collection (n = 414). The specimens were then taken for screening by osmotic fragility and IC strip test to specify alpha-thalassemia carriers. Another set of the specimens was sent for testing using hemoglobin (Hb) typing for thalassemia and abnormal Hb carriers and using multiplex polymerase chain reaction for alpha-thalassemia-1 carriers diagnosis as a gold standard.
Results:
There were 27 cases found as positive for alpha-thalassemia including alpha-thalassemia-1 carriers, South East Asian type, alpha-thalassemia-1 carriers, Thai deletion type, HbH and Hb constant spring, which were 18, 2, 3, and 2 cases, respectively. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of IC strip test were 92.6%, 95.1%, 56.8%, 99.4%, and 94.9%, respectively, which were higher than those of OFT.
Conclusions:
Accuracy of IC strip test was better than those of OFT. IC strip test may probably be very helpful in a massive thalassemia screening program.
Keywords
Alpha-thalassemia-1 carrier
immunochromatographic strip
one tube osmotic fragility test
thalassemia screening test
INTRODUCTION
Thalassemia is forms of inherited autosomal recessive blood disorders, which are characterized by the deformability and destructive red blood cells. Thalassemia is caused by variant or missing genes that affect to hemoglobin production. Thalassemia patients or carriers are less hemoglobin (Hb) concentration and lower circulating red blood cells in amount and size than normal, which results in mild to severe anemia. In Thailand, there are several types of thalassemia causing severe and complexity in pathological lesion starting from asymptomatic to very serious condition like perinatal mortality.[12]
It is estimated that in Thailand, there are about 24 million people who are thalassemia and abnormal Hb carriers with about 1% or 630,000 people show severe thalassemic syndrome. Every year, the increasing number of patients with thalassemia disease is about 4,253 cases resulting in the treatment expense of 21,487 million baht or about 700 million US dollar. Thalassemia is a serious problem for both medical and economic aspects. The Ministry of Health announced the policy for the prevention and control of thalassemia and abnormal Hb in Thailand saying that it was the fundamental right for every pregnant women and spouses to ask for screening diagnosis to see whether there was a chance to give a birth with a severe thalassemic syndrome child. Every community health center has to provide this screening test with the same standard.[23]
With the implementation of this policy, the number of specimens for thalassemia and abnormal Hb diagnosis increase significantly. Molecular diagnostic test can tell the mechanism of thalassemia disease and its severity accurately. However, the molecular diagnostic test is very expensive and complicated. With a simpler and more appropriate method, the diagnosis for local level will be more effective.[45]
The problem is to find the appropriate diagnosis or screening test with a simple, cheap, and practical tool for any staff to use. The objective of this test is to screen out people with a negative result and does not need to be tested with any other method. This is because this group of people who are alpha-thalassemia carriers will show moderate symptom if they are married with people with other types of thalassemia genes. People with the positive screening result do not mean that they are the certain carriers. It is necessary to do other types of diagnosis test to define the types of the carriers. The mentioned simple screening test may get a high chance to report false positive especially with iron deficiency anemia giving the positive result by an osmotic fragility test (OFT) similar to those with alpha-thalassemia-1 trait and beta-thalassemia trait.
Nowadays, the most convenient technique to screen alpha-thalassemia carriers is one tube OFT.[6] This technique can be used to screen alpha-thalassemia carriers in local remote area without any lab. However, there might be the number of false negative results from this type of the test especially with heterozygous beta-thalassemia, heterozygous alpha-thalassemia-1, and homozygous HbE which theoretically should not occur with practice error, such as mistake of laboratory record. Polymerase chain reaction (PCR) is a complicated method requiring too many kinds of tools and high expenses. Hence, it is not suitable for screening the high number people in a remote area without lab. Immunochromatographic (IC) strip test is simple with low cost similar to one tube OFT but more sensitivity (100%), high accuracy (98%) with only 3 min-diagnosis process. It does not require any special tool or any high skill staff in diagnosis and can detect specific Hb Bart's without reacting with other types of hemoglobin.[7]
According to the statistics from the laboratories in Kudjab, Udornthani Province in 2010, it was found that one tube OFT could reveal 83 positive cases from the sample of 427 people. Subsequently, those positive cases were retested with a standard method; it was found that only 24 cases were alpha-thalassemia carriers. The use of IC strip test will give more reliable results by decreasing the false negative results than that of one tube OFT. Moreover, it can also reduce the false positive results due to iron deficiency anemia; thus, reducing the expenses on confirmatory standard test.
With the problems mentioned above, the researchers were interested to compare the effectiveness and the accuracy of IC strip test (GPO α THAL IC strip test) and one tube OFT which are 2 popular methods in screening for thalassemia among pregnant women and their spouses with the confirmation method by high performance liquid chromatography (HPLC) and PCR to use as a guideline in selecting appropriate thalassemia screening program for local community.
SUBJECTS AND METHODS
Subject and blood collection
The project of ethylenediaminetetraacetic acid (EDTA) blood collection of pregnant women and their husbands at Kudjab Hospital, Udornthani Provinces between January and June 2013 was approved by Ethical Human Research Committee of Kudjab Hospital with MOU certification between Rajabhat Suan Sunadha University and the hospital, and all subjects gave written consent. The document details included names, surnames, admission number (AN), hospital number (HN), ward, and collecting time attached with the vacuum tubes as well as identifying those details in a request form.
Laboratory assay
Alpha-thalassemia screening program
-
Alpha-thalassemia screening was conducted by one tube OFT using KKU-OF (PCL Holding, Thailand) test set. The solution quality test was used to detect the thalassemia carrier type or EDTA blood with mean corpuscular volume (MCV) 60–70 fL and Hb > 12 g/dL as positive control or MCV > 80 fL and Hb > 12 g/dL as negative control. Then, EDTA blood test was done by dropping 20 μL of blood mixing with the solution and left for about 15 min before reading the result. The hemolysis of alpha-thalassemia carrier was not complete resulting in sediment solution while clear in normal people
-
Alpha-thalassemia screening was conducted by IC strip test (GPO α THAL IC strip test, Thailand) based on the principle that the patient's blood or thalassemia carrier's blood would contain Hb Bart's resulting in positive result on test strip. This can be explained that the protein of Hb Bart's in blood cells will be absorbed and reacted with antibody Hb Bart's attached on the test strip resulting in antigen-antibody complex. It then, permeated and reacted with anti-Hb Bart's antibody on the test strip.
The blood test was done by dropping 200 μL of blood in the test tube; then, added 200 μL of lysis buffer in the vortex mixture getting total 400 μL sample. The test strip should be used immediately at room temperature after opening by dipping it in the blood sample not exceeded the maximum indicator line and left it for 2–5 min before washing with washing buffer. The result should be read within 10 min.
Alpha-thalassemia carrier confirmatory test
-
Hemoglobin typing was used to distinguish thalassemia from abnormal Hb and deficiency anemia by preparing hemolysate; then analyzed the hemolysate using automatic VARIANT™ II TURBO (BIO-RAD, USA) based on HPLC of positive ion exchange. The dissolved blood was injected into the 2-headed analytical cartridge pump, concentrated buffer solution was also then injected into the cartridge where different types of Hb would be separated passing through the flow cell of filter photometer. Light absorption value was measured at the wavelength of 415 nm at the light filter of 690 nm. The light absorption value was interpreted and presented indifferent types Hb chromatogram retention time. However, this type of test could not confirm alpha-thalassemia disease. Hence, it was necessary to confirm with PCR-DNA analysis technique
-
Multiplex PCR: The detection of alpha-thalassemia-1 gene of both South East Asian (SEA) deletion (frequently founded) and THAI deletion (infrequently founded) to prevent Hb Bart's hydrops fetalis from homozygous alpha-thalassemia-1 could be carried out together with multiplex PCR. It could start by extracting DNA from the sample with Nucleo Spin Blood kit (Mecherey-Nagel, Germany); then, add DNA number using Applied Biosystems 7500 Real-Time PCR System (Life Technologies, USA) according to the manual of the company[8] while the primer and PCR condition were done by a test kit of Test Kit Center, Department of Medical Sciences[9] similar to general detecting of alpha-thalassemia-1 gene diagnosis. The technique of relative quantitative PCR has been designed with Primer and 3 Probes 3 differently labeled with illuminated substance. Probe RDP19 was specified with allele of α-globin gene was labeled with FAM color. Probe RD3 was specified with allele of abnormal alpha-thalassemia-1 SEA type labeled with NED color. Probe RD6 was specified with allele of abnormal alpha-thalassemia-1-Thai labeled with VIC color. In increasing heredity substance, 3 pairs of Primer 3, that is, Primers RD1 and RD2 were used for increasing DNA with abnormal alpha-thalassemia-1 SEA while Primers RD4 and RD5 were for increasing abnormal DNA of alpha-thalassemia-1 Thai. And the last pair, that is, Primers RD12 and RD13 were used for increasing normal DNA of α-globin gene. Those reacted with PCR using TaqMan Universal PCR Master Mix (Applied Biosystems, USA) in the authentic heredity substance increasing machine, model 7500 (Applied Biosystems, USA). DMSc α-thal 1 kit was a solution set for detecting alpha-thalassemia-1 (SEA and Thai [using Relative Quantitative PCR. This set consisted of 3 pairs of Primers 3 and 3 Probes] with the concentration of 10 pmol/μL each). Primer RD1 and RD2 are Primers for increasing DNA with abnormal alpha-thalassemia-1 SEA (SEA deletion). Primer RD3 was Probe specified for allele with abnormal alpha-thalassemia-1 SEA. Primer RD4 and RD5 were Primers for increasing DNA with abnormal alpha-thalassemia-1 Thai (Thai deletion). Primer RD6 was Probe specified for allele with abnormal alpha-thalassemia-1 Thai. Primer RD12 and RD13 were Primers for increasing DNA with normal α-globin gene. Primer RDP19 was Probe specified for allele with normal α-globin gene. The calculation of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and efficiency between IC strip test and OFTs were done by following formulae (TP = number of true positive; TN = number of true negative; FP = number of false positive and FN = number of false negative):
-
%sensitivity = (TP/TP + FN) × 100
-
%specificity = (TN/TN + FP) × 100
-
%PPV = (TP/TP + FP) × 100
-
%NPV = (TP/TP + FP) × 100
-
%efficiency = (TP + TN/TP + FP + TN + FN) × 100.
-
RESULTS
There was an alpha-thalassemia screening test conducted by one tube OFT, using a solution test kit, KKU-OF and IC strip test (GPO α THAL IC strip test) during January to June 2013 of 414 samples. IC of positive for alpha-thalassemia-1 carriers and normal were two bands and one band [Figure 1], respectively. The abnormal screening blood samples were confirmed for Hb analysis using HPLC and to detect alpha-thalassemia-1 (SEA and Thai deletion) by using Relative Quantitative PCR to find false positive values and false negative values of both methods. Hb typing was used to distinguish normal, thalassemia and other types of Hb abnormalities [Figure 2] especially between beta-thalassemia, heterozygous HbE, (EA) and homozygous HbE (EE) and also to diagnose HbH and Hb constant spring (HbCS) carriers, however, it was difficult to interpret and need expert laboratory technician for confirmed the chromatographic results. The detection of alpha-thalassemia deletion using multiplex PCR technique was separated PCR products by agarose gel electrophoresis, which were interpreted band pattern on each lane when compared to DNA ladder [Figure 3]. The result revealed that among the 414 samples in this study, there were 27 cases found as positive for alpha-thalassemia including 18 of alpha-thalassemia SEA type (SEA deletion), 2 of Thai type (Thai deletion), 2 of SEA type together with Thai type, 3 of HbH (HbH with alpha-thalassemia-1/alpha-thalassemia-2) = 1 sample, HbH with HbE trait = 1 sample and HbH and HbCS with HbE trait = 1 sample and 2 of HbCS as shown in Table 1. Those thalassemia carriers screened in this study by KKU-OF and GPO α THAL IC strip test showed negative results for 12 cases and 2 cases, respectively. However, when the negative samples were retested for the confirmation with multiplex PCR, the results were negative. It can be concluded that the KKU-OF and GPO α THAL IC strip tests reported false negative, FN, 12 and 2 cases, respectively. In detecting alpha-thalassemia carrier, there were 36 cases of HbE and beta-thalassemia trait; 21 cases were homozygous HbE (EA), 13 cases were heterozygous HbE (EA) and 2 cases were beta-thalassemia trait which were abnormal β-Hb or false positive, FP. KKU-OF and GPO α THAL IC strip test reported 12 and 2 cases of false positive results respectively. The report of positive, negative, false positive, and false negative of thalassemia screening detection is shown in Table 1.

- Immunochromatograph (IC) of IC strip (GPO α THAL IC strip test, Thailand) for α-thalassemia screening carriers. The left strip and right strip were presented two bands and one band of ICs, which were interpreted as positive and negative for alpha-thalassemia-1 carriers, respectively
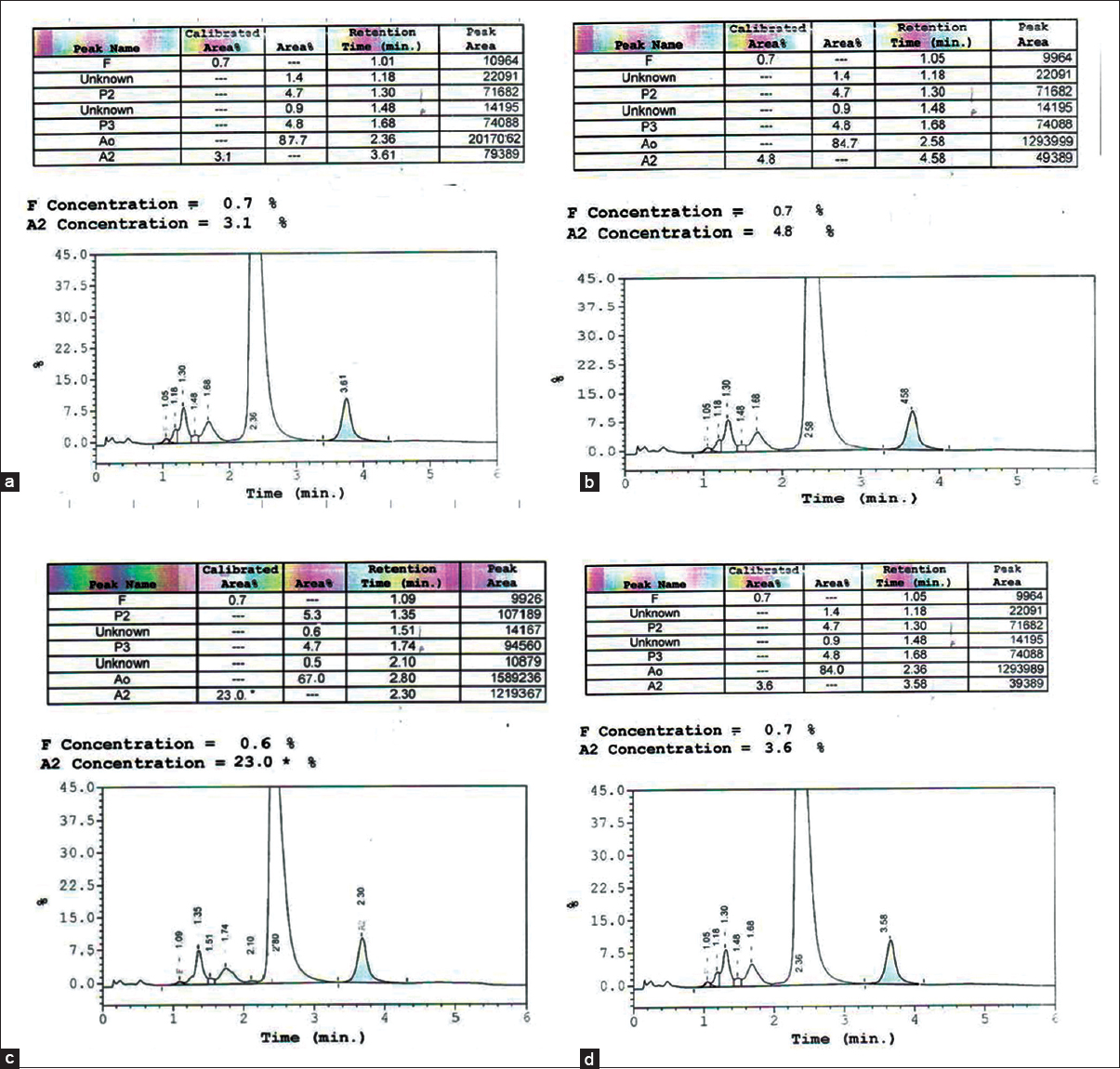
- Chromatogram of hemoglobin (Hb) typing by high-performance liquid chromatography. The results read as follows: (a) A2A; (b) A2A (increased A2 than normal); (c) EA; (d) EE, which were interpreted as normal, beta-thalassemia trait, heterozygous HbE, and homozygous HbE, respectively
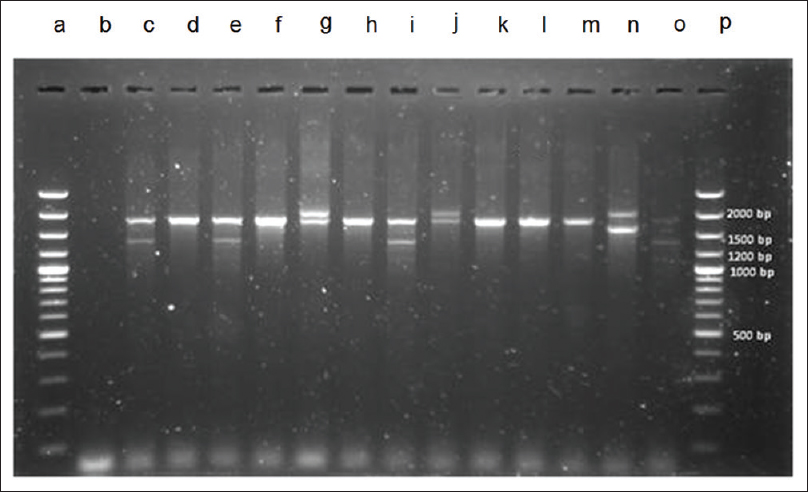
- DNA electrophoresis separation of polymerase chain reaction (PCR) products from multiplex PCR. (1) Lane a and p were DNA ladder. (2) Lane d, f, h, k, l, and m were interpreted to normal globin gene (1,800 bp). (3) Lane c, e, i, and o were interpreted to South East Asian type. (4) Lane g and j were interpreted to 3.7 kb deletion type (2,022 bp). (5) Lane n (control) was 4.2 kb deletion type. Except DNA ladder, all lanes must be 1 band to 2 bands because alpha globin cluster had only 2 alleles

When TN, TP, FN, and FP were calculated [Table 2] to find sensitivity, specificity, PPV, NPV, and efficiency of test/accuracy, it was found that IC strip showed higher values than those of KKU-OF as shown in Table 3. However, the PPV of KKU-OF was 33.3% while IC-strip was relatively low at 56.8% due to the false positive from HbE and beta-thalassemia trait. This study evaluated the efficiency of the 2 methods for screening alpha alpha-thalassemia carrier; IC strip and one tube OFT (KKU-OF). The results showed that sensitivity of test, specificity of test, PPV, NPV, and efficiency of test/accuracy of IC strip were 92.6, 95.1, 56.8, 99.4, and 94.9%, respectively. These were higher than the results of sensitivity of test, specificity of test, PPV, NPV, and efficiency of test/accuracy of KKU-OF at 55.5, 92.2, 33.3, 96.7, and 89.8%, respectively.


DISCUSSION
Identification of a-thalassemia 1 carriers is an important step toward the prevention of Hb Bart's hydrops fetalis (homozygous a-thalassemia 1), which is the most severe thalassemia disease. Of the various methods used to identify a-thalassemia 1 carriers, PCR is the most reliable and accurate and is currently the gold standard.[1011] However, certain significant limitations such as cost, the need for sophisticated laboratory instrumentation combined with well-trained technicians currently prevents PCR from being widely used for a-thalassemia 1 screening especially for mass screening in rural area or community hospital. A simple and fast qualitative screening test that can be performed at the place of sampling is required to achieve the effective surveillance of α-thalassemia 1 carriers.[11] By this point, the screening test is most important to prevent or control thalassemia in developing countries, which were limited in financial and expert laboratory technician supports.
In this study, it can be seen that sensitivity of test and PPV of KKU-OF were considerably lower than those of IC strip. This can be explained that the principle of IC strip was based on Hb Bart's which was higher specificity and no reaction with other types of Hb. The study by Wanapirak and others[7] showed that the sensitivity and accuracy of IC strip were 100% and 98%, respectively, which were much higher than the results of this study with similar samples (n = 499). This might be due to the decreasing results of sensitivity and specificity giving the false negative through IC strip screening. However, one case of positive alpha-thalassemia carrier became negative through PCR, might be because the quantity of Hb Bart's in blood stream of some carriers were very low[10] and E type Hb especially heterozygous HbE (EE) could give false positive value through IC strip as well which might result from the anemia of E type Hb with lower hematocrit (Hct) values (reference values: male Hct = 40.7–50.3%, female Hct = 36.1–44.3%). When we examined the blood sample from anemia persons with low Hb quantity to detect alpha-thalassemia-1 through IC strip, the results might be unclear and need to be retested for confirmation. Moreover, the staff's skill also affects the interpretation and the effectiveness of the screening test as well.
The KKU-OF method was based on osmotic fragility principle that is to say the increase osmotic fragility or decrease osmotic fragility depended on the ratio of cell wall area to the concentration of cell internal substances which most of them were Hb. When there was a ratio of Hb quantity, there was the decrease of osmotic fragility such as target cell (found in thalassemia carrier, Thalassemia disease, and hepatitis), and hypochromic cell (found in Thalassemia and anemia). When spherocyte decreased (cell wall area decreased), the Hb would be more fragile. With KKU-OF method, there was a high chance to get the result in false positive due to iron deficiency anemia which the result from OFT will be positive similar to those who were HbE and beta-thalassemia trait resulting in lower specificity value of KKU-OF method. Moreover, the sensitivity of KKU-OF was lower than that of IP strip due to the negative false value of one tube OFT. Theoretically, the false negative value from the heterozygous beta-thalassemia, heterozygous alpha-thalassemia-1, and homozygous HbE should not occur, however, in practice, errors could be caused by several reasons such as the analyst and data input. The suggestion was on the use of KKU-OF together with 2,6 dichlorophenol indophenols (DCIP) test based on the principle that DCIP colors would make sediment on unstable Hb such as E-Hb and H-Hb. When using this method with 0.36% NaCl OFT, it would result in easier screening interpretation especially when using in local remote area. This method could differentiate between alpha-thalassemia carriers from E-Hb. However, DCIP test method had some problems on result display that blue color made it difficult to see the sediment and needed decolorization or light box or solid line written for easier reading.[12] Moreover, DCIP test should be done at particular temperature and accuracy incubation time to avoid negative false. Furthermore, DICP is an oxidizing agent which could be deteriorated if keeping for a long period. PCR technique was a global standard technique to detect α-thalassemia-1 carriers with high sensitivity and specificity.[11131415] However, some limitations of PCR were high cost and high skill staff requirement. The screening method for detecting thalassemia carriers and abnormal Hb in Thailand should be simple, cheap, and require no complicated tools which could be carried out in local remote health centers. The main purpose of the screening was to screen out the negative case requiring no other retest methods. IC strip should be a good alternative to the previous ones.[710] It could be concluded that IC strip method resulted in higher accuracy than that of KKU-OF but could not be used for the confirmation as the alternative for PCR. This would be useful for screening the high number of population. In combination with red blood cell indices, the IC strip test could rule out mass populations for further α0-thalassemia detection by PCR-based analysis. The alpha thalassemia IC strip also has the potential to replace testing for HbH inclusion bodies, as it appears to be more sensitive, specific, and less labor intensive.[16]
Moreover, the use of IC strip with other screening methods like MCV (normal range = 100–80 fL) in daily complete blood count would yield in more effective results.
ACKNOWLEDGMENTS
We are grateful to Suan Sunandha Rajabhat University, Bangkok, Thailand for grant support. We would like to sincerely thank all staffs of Division of Pathology, Kudjab Hospital located in Udonthani Province, Thailand for a research facility and all volunteers for providing useful data and helping on this research.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Haemoglobinopathies including thalassaemia. Part 1: Tropical Asia. Clin Haematol. 1981;10:707-29.
- [Google Scholar]
- Thalassemia in SouthEast Asia: Problems and strategy for prevention and control. Southeast Asian J Trop Med Public Health. 1992;23:647-55.
- [Google Scholar]
- A reliable screening protocol for thalassemia and hemoglobinopathies in pregnancy: An alternative approach to electronic blood cell counting. Am J Clin Pathol. 2005;123:113-8.
- [Google Scholar]
- Screening for thalassemia: A model of success. Obstet Gynecol Clin North Am. 2002;29:305-28. vi
- [Google Scholar]
- Prenatal control of severe thalassaemia: Chiang Mai strategy. Prenat Diagn. 2000;20:229-34.
- [Google Scholar]
- Wintrobe's Clinical Hematology. (9th ed). Philadelphia USA: Lee and Febeiger; 1993.
- [Google Scholar]
- Accuracy of immunochromatographic strip test in diagnosis of alpha-thalassemia-1 carrier. J Med Assoc Thai. 2011;94:761-5.
- [Google Scholar]
- Life Technologies Corporation. Real-time PCR Handbook. USA: Life Technologies Corporation; 2012.
- [Google Scholar]
- Test Kit Center. Department of Medical Sciences. 2011. Available from: http://www.dmsc.moph.go.th/testkit
- [Google Scholar]
- Simple method for screening of alpha-thalassaemia 1 carriers. Int J Hematol. 2009;89:559-67.
- [Google Scholar]
- Rapid diagnosis of alpha-thalassemia-1 of southeast Asia type and hydrops fetalis by polymerase chain reaction. Blood. 1991;78:853-4.
- [Google Scholar]
- Reassessment of a simple chemical method using DCIP for screening for haemoglobin E. J Clin Pathol. 2006;59:74-6.
- [Google Scholar]
- A simplified screening for alpha-thalassemia 1 (SEA type) using a combination of a modified osmotic fragility test and a direct PCR on whole blood cell lysates. Acta Haematol. 2002;108:74-8.
- [Google Scholar]
- Development and application of a real-time quantitative PCR for prenatal detection of fetal alpha (0)-thalassemia from maternal plasma. Ann N Y Acad Sci. 2006;1075:103-7.
- [Google Scholar]
- Detection of alpha-thalassemia in beta-thalassemia carriers and prevention of Hb Bart's hydrops fetalis through prenatal screening. Haematologica. 2006;91:649-51.
- [Google Scholar]
- Validation of the immunochromatographic strip for α-thalassemia screening: A multicenter study. Transl Res 2014 doi: 10.1016/j.trsl.2014.10.013. [Epub ahead of print]
- [Google Scholar]





