Translate this page into:
Epidemiology of Rifampicin Resistant Tuberculosis and Common Mutations in rpoB Gene of Mycobacterium tuberculosis: A Retrospective Study from Six Districts of Punjab (India) Using Xpert MTB/RIF Assay
Address for correspondence: Dr. Neerja Jindal, E-mail: neerjarajender@hotmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Xpert MTB/RIF assay has revolutionized the diagnosis of tuberculosis (TB) by simultaneously detecting the bacteria and resistance to rifampicin (RIF), a surrogate marker for multidrug-resistant TB (MDR-TB) in <2 h. The RIF resistance pattern in Malwa region of Punjab, India, is not documented. Here, we report the epidemiology of RIF-resistant TB and mutations in rpoB gene of Mycobacterium tuberculosis (MTB).
Materials and Methods:
A total of 1612 specimens received between October 2013 and February 2015 were tested by Xpert MTB/RIF assay following manufacturer's instructions. The results thus obtained were analyzed using SPSS version 20.0.0 (SPSS Inc., Chicago, IL, USA) statistical software.
Result:
RIF resistance was statistically higher in previously treated patients in comparison to the new patients (P = 0.006) and in patients with acid fast-Bacilli (AFB) positive smears to AFB-negative smears (P = 0.048). RIF resistance mutations in 130 specimens revealed frequency of E 73/130 (56%), B 28/130 (21.5%), D 18/130 (13.8%), A 11/130 (8.4%), and C 1/130 (0.7%) while in one specimen, mutation combination, i.e., mutations associated with more than one probe (A and B both) was present.
Conclusion:
Xpert MTB/RIF assay is a user-friendly screening tool for detection of MTB and RIF resistance from suspected TB/MDR cases in a shorter period of time. It could also serve as a useful technique to have simultaneous preliminary information regarding the mutation pattern of RIF resistance in MTB isolates.
Keywords
Epidemiology
multidrug-resistant tuberculosis
Mycobacterium tuberculosis
rifampicin resistance determining region
Xpert MTB/RIF assay
INTRODUCTION
With the worldwide spread of Mycobacterium tuberculosis (MTB) strains resistant to both isoniazid and rifampicin (RIF), multidrug-resistant (MDR) tuberculosis (TB)[1] has become a major public health problem posing formidable challenges due to its complex diagnostic and treatment requirements. This highlights the need for having rapid molecular diagnostic techniques which could help facilitate early diagnosis and appropriate delivery of anti-tubercular therapy. Xpert MTB/RIF assay (Cepheid), a real-time automated nucleic acid amplification system is one such technique which detects MTB as well as mutations that confer resistance to RIF in >2 h. It was installed in our institute (Guru Gobind Singh Medical College and Hospital, Faridkot) in October 2013 under Revised National Tuberculosis Control Programme (RNTCP) TB Xpert project supported by WHO-STOP TB partnership-UNITAID.
RIF is a principle first line anti-TB drug and RIF resistance is a surrogate marker for MDR-TB. The genetic basis of RIF resistance (in approximately 95% cases) is the presence of mutations in 81 bp RIF resistance determining region (RRDR) of the rpoB gene, corresponding to codons 507–533 (Escherichia coli numbering system), which codes for a beta subunit of RNA polymerase of MTB.[2] Studies conducted in diverse geographical areas have shown that the burden of MDR-TB and the mutations responsible for drug resistance vary from country to country and region to region.[3] However, there is not enough of information about this important aspect from North India and the data from Malwa region of Punjab is almost lacking. Therefore, this retrospective study was conducted to determine the frequency of RIF resistance and rpoB gene mutations among the suspected TB/MDR cases of Malwa region of Punjab (districts: Faridkot, Fazilka, Firozpur, Moga, Bathinda, and Muktsar-area approximately 14,981 km2), using Xpert MTB/RIF assay. Knowledge of the pattern of mutations present in RIF-resistant isolates could provide insight into the epidemiology of RIF-resistant MTB isolates of this particular area.
MATERIALS AND METHODS
A total of 1612 sputum specimens which originated from patients with suspected MDR pulmonary TB of Malwa region of Punjab were received in microbiology laboratory, under RNTCP for Xpert MTB/RIF assay between October 2013 and February 2015. The samples were subjected to Ziehl-Neelsen (ZN) staining and Xpert MTB/RIF assay. Grading of ZN stained smears was done according to RNTCP guidelines. The Xpert MTB/RIF assay was performed directly on sputum specimens[45] using the Xpert MTB/RIF assay G4 version 5 and the software version 4.4a as per the manufacturer's instruction (Cepheid, Sunnyvale, CA, USA). Briefly, it consisted of inactivation of the sputum specimen with NaOH and isopropanol (sample reagent) used in 1:2 ratio for 15 min. The mixture was then introduced into the Xpert MTB/RIF cartridge and loaded into the Xpert MTB/RIF instrument for DNA extraction and amplification of 192 bp segments of the rpoB gene. The detection consists of hybridization of the amplicon with five overlapping probes complementary to the rpoB “core” region (81 bp) determining the RIF resistance.[6] The results along with graphs were available in 1 h 45 min. The reports were communicated electronically to the concerned district TB officer and drug-resistant TB center.
Statistical analysis
Chi-square test of proportions was used to identify a significant difference between two or more groups, and P < 0.05 was considered as statistically significant. To determine the association of various epidemiological characteristics of the patients to RIF-resistant TB, odds ratio (OR) and 95% confidence interval (CI) were calculated using SPSS statistical software version 20.0.0 (SPSS Inc., Chicago, IL, USA). The relationship of smear positivity grade with the relative bacterial burden and Xpert MTB/RIF Ct value (given in GeneXpert Operator's Manual by Cepheid) was also analyzed.
RESULTS
Study population
Out of 1612 specimens tested, 1308 were positive for MTB by Xpert MTB/RIF assay. There were 34 “errors” and 18 specimens showed “no results.” All these specimens were retested, and valid results were obtained except in two (one showed error in the second sample also and another could not be processed due to insufficient material in the second specimen).
ZN staining of the 1308 specimens positive for MTB by Xpert MTB/RIF assay showed that 1240 were positive for acid fast-Bacilli (AFB) with 272 (21.9%) being grade 3 positive. Correlation of smear positivity grade with relative bacterial burden and Ct value showed that out of total 272 grade 3 positive specimens, 69.4%, 22.5%, 6.5%, and 1.6% had high (Ct <16), medium (Ct 16–22), low (Ct 20–28), and very low (Ct >28) bacterial load, respectively. Of the specimens showing Grade 2, Grade 1 scanty positivity, the maximum belonged to high and medium, high and medium, and medium bacterial load groups, respectively. In sputum negative specimens (68/1308) the maximum number belonged to low bacterial load group.
Of 1308 MTB-positive samples by Xpert TB/RIF assay, 130 (9.9%) demonstrated RIF resistance. RIF resistance “Indeterminate” results were obtained in 4 specimens and on retesting 2 came out to be RIF sensitive. The other two could not be retested due to the specimens being inadequate. All these 4 specimens were smear-positive and had Ct value was >28.
The study of the association of epidemiological characteristics of the patients to the RIF-resistant TB showed that RIF resistance was present in statistically significant higher number in previously treated patients in comparison to the new patients (95% CI 1.148–7.135, P = 0.006 and OR = 2.86) and in patients with AFB-positive sputum smears to AFB-negative smears (95% CI 0.911–15.5, P = 0.048 and OR 3.7). However, the difference was found to be statistically insignificant between the different age groups of patients (P = 0.053), male and female patients (95% CI 0.706–1.57, P = 0.798 and OR 1.054), patients from rural and urban background (95% CI 0.69–1.49, P = 0.918 and OR 1.02) and HIV-positive and HIV-negative patients (95% CI 0.219–4.13, P = 0.949 and OR 0.953).
The study of mutation pattern of RIF resistance in 130 specimens revealed that in 129, rpoB gene mutations in 81 bp RRDR of MTB were detected by one of the 5 different probes (A, B, C, D, E), while in one specimen, mutation combination, i.e. mutations associated with more than one probe (A and B both) was present. The probe frequencies associated with the observed RIF resistance were as follow: E 73/130 (56%), B 28/130 (21.5%), D 18/130 (13.8%), A 11/130 (8.4%), and C 1/130 (0.7%) [Table 1]. Accordingly, the frequencies of mutations at 5 different rpoB gene regions were 529–533 (56%), 512–518 (21.5%), 523–529 (13.8%), 507–511 (8.4%), and 518–523 (0.7%).
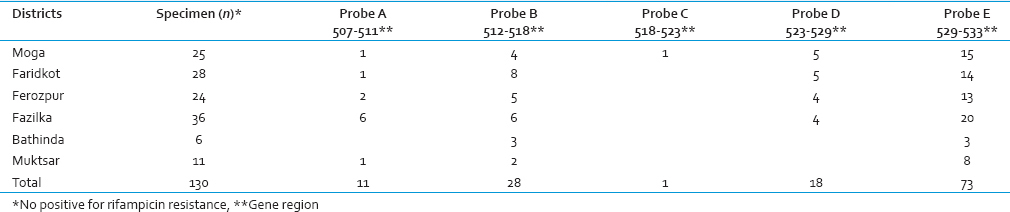
Correlation of mutations with the RIF-resistant sputum specimens obtained from different districts [Table 1] revealed that probe E which represented mutation in region 529–533 was the most common in all the six districts of the study. Only one specimen revealed RIF resistance by probe C depicting mutation in codon region 518–523.
DISCUSSION
Although, India ranks first out of the 22 countries with the highest burden of TB, but the reliable information on the magnitude of MDR-TB in the country is largely unavailable.[7] By using Xpert MTB/RIF assay, we observed RIF resistance which is a surrogate marker of MDR-TB in 9.9% of suspected cases of MDR-TB of Malwa region of Punjab. These findings are similar as reported from Jaipur (11.09%).[8] However, higher prevalence of MDR-TB has been reported in other Indian studies (Lucknow 27.8%,[9] New Delhi 17.9%,[10] and Central India 17%).[11]
Globally, 3.7% of new cases and 20% of previously treated cases are estimated to have MDR-TB. In India, the estimated figure in new cases with MDR-TB is 2.1% with CI 1.5–2.7 and estimated percentage in previously treated cases is 15% with CI of 13–17, respectively.[12] RNTCP carried out drug-resistant surveys in accordance with global guidelines and indicated a low prevalence of MDR-TB, i.e., >3% among new cases and 12–17% in previously treated cases in Gujarat, and Maharashtra and Andhra Pradesh.[13] In the present study, the figure was 3.9% (5/126) in the new and 10.6% (125/1182) in the retreatment cases, and the difference between the two was statistically significant (P = 0.006). Gupta et al. had also observed that MDR-TB was significantly higher in previously treated cases compared to new cases and concluded that the previous exposure to anti-TB agents was the most common cause of developing MDR.[14]
In the present study, another statistically significant association was seen between smear-positive MDR cases in comparison to smear-negative MDR cases (P = 0.048). Gupta et al. had also observed a higher but statistically insignificant association of MDR-TB with smear positivity.[14] This could be because all the MDR-TB patients of our study had pulmonary TB and these patients are more likely to have cavitary lesions and positive sputum smear results.
Our study population showed slightly higher prevalence of RIF resistance in HIV-negative patients than HIV-positive patients but statistically the difference was insignificant (P = 0.949). This is in contrast to the study of Gupta et al. who observed a statistically significant difference in MDR-TB in HIV-negative in comparison to a HIV-positive group of patients.[14] Quy et al. reported that the failure of treatment of MDR-TB was associated with MDR and not with HIV infection.[15]
Although statistically there were insignificant differences between different age groups showing MDR-TB (P = 0.053), a maximum number of cases in the present study were in the age group of 21–40 years. Other Indian studies have also reported the predominance of younger age group with MDR-TB.[10] There were also a higher number of male patients and patients from a rural background with MDR-TB in our study. This is similar to the findings of Gupta et al.[14] As young adult males are economically productive segment of our society, high MDR-TB in this group has several socioeconomic implications.
While detecting TB/MDR cases, Xpert MTB/RIF assay showed 2.1% (34/1612) errors in the present study. Mboowa et al. have reported 12% errors using Xpert MTB/RIF assay G3 version 3 and G4 version 5, with more than half being contributed by the former version.[16] The lower percentage of errors in the present study could be because that we used the upgraded G4 version 5 of Xpert MTB/RIF assay. Similar to our study, Rufai et al. have reported 1.8% errors using Xpert MTB/RIF assay G4.[17]
Nwokoye et al. have stated that low bacterial load limits the ability of the Xpert MTB/RIF assay to correctly identify mutated and wildtype sequences in the core region of the rpoB gene.[18] The “Indeterminate” results of RIF resistance detected in four samples of the present study could be because of their high Ct value (>28) corresponding to the very low bacterial burden.
While using Xpert MTB/RIF assay for the detection of MTB and RIF resistance in MDR suspected cases, we could collect additional information on mutations associated with RIF resistance. It was observed that the pattern of mutations in the 81 bp RRDR of MTB isolates of the present study was almost similar to that reported from other parts of India. The most common RRDR rpoB gene mutations were in the gene region 529–533 (56%; Table 1) in all the six districts of Malwa region and were recognized by probe E. Mboowa et al. too used Xpert MTB/RIF assay and the most common gene mutations observed were in codon 531 (58%) followed by 513 (25%), 526 (8%), and 511 (8%) designed by probes E, B, D, and A.[16] Singhal et al. used GenoType MTBDR plus assay and reported 531 as the most commonly mutated codon in 59.0%.[10] Similarly, Mani et al. reported that the codons most commonly involved in these mutations were 531 (53%) and 526 (19%)[19] in a study from South India. The resistant mutants isolated more frequently in clinical practice have higher mean relative fitness and their prevalence depend on their ability to survive.[20] This might be the reason for the higher occurrence of mutations in codon 531–533.
In our study, we observed RIF resistance by probe C (518–523) in one of the 130 RIF resistant sputum samples. This is similar to the findings of Gupta et al. who used DNA sequencing combined with MS-PCR assay to observe mutation in this rpoB region.[14] In contrast, no RIF resistance was found to be associated with probe C in the study of Mboowa et al.[16] This could probably be because of lesser susceptibility of this genetic region to mutations or because the selection pressure shaping (producing) probe C associated RIF resistance is less in Malwa region of Punjab (North India).
Mutation combination (probe A and B) was observed in one (1/130) of the RIF-resistant specimens in the present study. While, Singhal et al. found 6 strains (6/366) with more than one mutations[10] and Mboowa et al. reported no specimen with more than one probe failure (mutation combination) while employing Xpert MTB/RIF assay in their study.[16] Probably, mutations continue to arise due to the ability of MTB to adapt to drug exposure.[19]
The limitation of the study was that no gold standard was used for the comparison of Xpert MTB/RIF assay results.
CONCLUSION
Xpert MTB/RIF is a better screening tool for detection of MTB and RIF resistance in a shorter period of time, and this could help improve early recognization of MDR-TB and prevention of its further transmission in Malwa region of Punjab. This assay also appears to be a useful technique to have simultaneous preliminary information regarding the mutation pattern of RIF resistance in MTB isolates which could be helpful in understating the epidemiology of the disease and identification of hot spots for implementation of TB control program. However, for the confirmation and the detailed study on these mutations, DNA sequencing remains indispensable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Multi-drug resistant tuberculosis: An iatrogenic problem. Biosci Trends. 2010;4:48-55.
- [Google Scholar]
- Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber Lung Dis. 1998;79:3-29.
- [Google Scholar]
- Determination of drug susceptibility patterns and genotypes of Mycobacterium tuberculosis isolates from Kanpur district, North India. Infect Genet Evol. 2011;11:469-75.
- [Google Scholar]
- Xpert MTB/RIF assay for diagnosis of pulmonary tuberculosis in sputum specimens in remote health care facility. BMC Microbiol. 2015;15:220.
- [Google Scholar]
- Evaluation of the Xpert MTB/RIF test for the diagnosis of childhood pulmonary tuberculosis in Uganda: A cross-sectional diagnostic study. BMC Infect Dis. 2013;13:133.
- [Google Scholar]
- Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J Clin Microbiol. 2001;39:4131-7.
- [Google Scholar]
- World Health Organization. Global Tuberculosis Control: Epidemiology, Strategy, Financing. WHO Report 2009. Geneva: World Health Organization; 2009.
- [Google Scholar]
- Prevalence of MDR-TB and mutational pattern in rpoB gene of Mycobacterium tuberculosis at Eastern Rajasthan. Microcon 2014. (38th National Conference of Indian Association of Medical Microbiologist) . 2014;MP39:253.
- [Google Scholar]
- Declining trend of resistance to first-line anti-tubercular drugs in clinical isolates of Mycobacterium tuberculosis in a tertiary care North Indian hospital after implementation of revised national tuberculosis control programme. Indian J Med Microbiol. 2014;32:430-3.
- [Google Scholar]
- Early detection of multi-drug resistance and common mutations in Mycobacterium tuberculosis isolates from Delhi using GenoType MTBDRplus assay. Indian J Med Microbiol. 2015;33(Suppl (1)):46-52.
- [Google Scholar]
- Clonal diversity and drug resistance in Mycobacterium tuberculosis isolated from extra-pulmonary samples in central India – A pilot study. Indian J Med Microbiol. 2014;32:434-7.
- [Google Scholar]
- Declining trend of resistance to first-line anti-tubercular drugs in clinical isolates of Mycobacterium tuberculosis in a tertiary care north Indian hospital after implementation of revised national tuberculosis control programme. Indian J Med Microbiol. 2014;32:430-3.
- [Google Scholar]
- TB India 2012, Annual Status Report Government of India. 2012. Available from: http://www.tbcindia.com
- [Google Scholar]
- Association of MDR-TB isolates with clinical characteristics of patients from Northern region of India. Indian J Med Microbiol. 2014;32:270-6.
- [Google Scholar]
- Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2006;10:45-51.
- [Google Scholar]
- Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert® MTB/RIF in Kampala, Uganda: A retrospective study. BMC Infect Dis. 2014;14:481.
- [Google Scholar]
- Performance of Xpert MTB/RIF assay in diagnosis of pleural tuberculosis by use of pleural fluid samples. J Clin Microbiol. 2015;53:3636-8.
- [Google Scholar]
- Non-conforming rifampicin susceptibility test reports from Xpert MTB/RIF assay: The national reference laboratory experience in Nigeria. Open Sci J Clin Med. 2014;2:59-62.
- [Google Scholar]
- Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from India. J Clin Microbiol. 2001;39:2987-90.
- [Google Scholar]
- Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:1866-9.
- [Google Scholar]





