Translate this page into:
Etiology of Pancytopenia: An Observation from a Referral Medical Institution of Eastern Region of India
Address for correspondence: Dr. Senjuti Dasgupta, E-mail: dasguptasenjuti@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Pancytopenia is a relatively common hematological condition, the etiological factors of which vary widely in different geographic location. Determining the specific etiology is of immense importance for appropriate management.
Aims and Objectives:
The present study was undertaken to delineate etiological factors leading to pancytopenia in a Tertiary Care Hospital of West Bengal from Eastern Region of India.
Materials and Methods:
A prospective study was conducted for a period of 2 years in which 248 patients were included. After obtaining a relevant clinical history, physical examination was done followed by complete blood count including peripheral blood smears examination, relevant biochemical, and radiological investigations. Afterward, bone marrow aspiration and biopsy were performed and microscopically examined.
Results:
Among 248 patients studied, 156 (62.9%) were males and 92 (37.09%) were females. The mean age of the patients was 33 years. Aplastic anemia was the most common cause of pancytopenia that was observed in 83 cases (33.47%) followed by megaloblastic anemia in 52 cases (20.97%), leishmaniasis in 34 patients (13.71%), hypersplenism also in 34 patients (13.71%), and tuberculosis and other connective tissue disorders in 18 cases (7.26%). The occurrence of aplastic anemia was statistically significant in pediatric (≤15 years) age group.
Conclusion:
Aplastic anemia was found to be the most common cause of pancytopenia in this study, which is in contrast to studies conducted from other regions of India. Delineation of etiologies of pancytopenia in various regions can help in defining diagnostic and therapeutic strategies, which is expected to contribute toward the better management of such patients.
Keywords
Clinical features
etiology
hematological findings
pancytopenia
INTRODUCTION
Pancytopenia is a condition characterized by simultaneous presence of anemia, leukopenia, and thrombocytopenia. Clinical features of pancytopenia are usually consequences of these factors or their combination leading to life-threatening infection or bleeding in the late stages of disease. In routine institutional hematology practice, the condition is frequent.[1]
Various etiological factors have been implicated in the causation of pancytopenia. Major factors include reduction in blood cell production, infiltration of bone marrow (BM) by abnormal cells, suppression of BM, ineffective hematopoiesis with cell destruction, antibody mediated destruction of cells, and sequestration of cells in the reticulo-endothelial system.[2] Treatment and prognosis of patients with pancytopenia are governed by the cause and severity of the underlying disease.[3]
Variation in etiology of pancytopenia is not only appreciated in different countries but also in different regions of a single country. Studies from North and South India have implicated megaloblastic anemia as the most common cause of pancytopenia, whereas a study conducted in Maharashtra has found hypersplenism and infections to be the most frequently responsible diseases.[456] Aplastic anemia and erythroid hyperplasia were found to be the most common cause of pancytopenia in a study was undertaken in Nepal.[7] Aplastic anemia followed by infections such as malaria and leishmaniasis were the major causes of pancytopenia reported from Bangladesh.[8] In contrast, neoplastic diseases and radiation have been reported as the most common cause of pancytopenia, in Europe and Israel.[9] Similar data on etiology of pancytopenia from Eastern Region of India is lacking in the literature. Documentation of variation in etiology of pancytopenia has an important role in formulating a comprehensive diagnostic and therapeutic strategy, unique to pancytopenic patients of a particular region. This is instrumental in the delivery of early and appropriate management. The present study was conducted to find out the causes leading to pancytopenia, their distribution among the study cohort and their clinico-pathologic features.
MATERIALS AND METHODS
This study was conducted in the Department of Pathology of a Tertiary Care Medical Teaching Institution from Eastern Region of India for a period of 2 years. During this time, patients who fulfilled the following criteria were provisionally selected in the study: Simultaneous peripheral blood hemoglobin level <13.5 g/dl in males, or 11.5 g/dl in females, leukocyte count <4 × 109/L, and the platelet count <150 × 109/L.[1] Patients with iatrogenic cause (e.g., drugs, radiation) or infections (e.g., septicemia, dengue, malaria) were excluded from the present study.
A signed consent form was obtained from all the patients included in the study. A detailed clinical history was noted from each patient followed by a thorough physical examination. Specific features in respect of symptoms related to pancytopenia such as fatigue, palpitation, shortness of breath, fever, and easy bruising were enquired. A general survey of each patient was followed by a thorough systemic examination. Chest radiography and ultrasonography of abdomen were undertaken to note the condition of different organs.
Blood samples were collected for complete blood count (CBC) and peripheral blood smear (PBS) examination. The smear was stained by Leishman stain for PBS examination and by new methylene blue to note the reticulocyte count. The blood collected in the ethylenediamine tetraacetic acid vial was analyzed with Sysmex KX21 automated cell counter to obtain the CBC. Clotted blood sample was also collected from each patient for the following biochemical investigations: Liver function tests, serum urea, creatinine, fasting glucose, serum iron, and ferritin. Serum Vitamin B12 and folate levels were assayed by using Beckman coulter access 2 immunoassay system.
BM aspiration (BMA) and trephine biopsy were then performed using Salah's BMA needle and Jamshidi needle, respectively. BMA smears were stained with Leishman stain. Touch imprints of the biopsy specimen were made, which were also stained with Leishman stain. The biopsy specimens were collected in 10% neutral buffered formalin. The specimen was then processed to obtain paraffin wax blocks. Thin sections (<4 µm) were cut using the microtome, and these were stained with hematoxylin and eosin stain. The BMA smears and the biopsy sections were studied in detail.
As and when required, BMA and BM biopsy slides were stained with Perl's stain, periodic acid Schiff stain, Zeihl–Neelsen stain or Reticulin stain, other relevant tests such as the test for rheumatoid arthritis (RA) factor and protein electrophoresis were done in appropriate cases.
Relevant data were recorded, tabulated and analyzed.
RESULTS
A total 248 patients with pancytopenia were included in the study group. Of these patients, 156 (62.9%) were males and 92 (37.09%) were females (male: female = 1.7:1). The age of these patients ranged from 3 to 84 years with a mean age of 33 (Standard deviation [SD] ±18 years).
The most common cause of pancytopenia was found to be aplastic anemia, present in 83 patients (33.47%) followed by megaloblastic anemia in 52 patients (20.97%). Other common causes included leishmaniasis and hypersplenism in 34 patients each (13.71%), tuberculosis in 12 patients (4.84%), and other connective tissue disorders in 6 cases (2.42%) cases [Table 1].
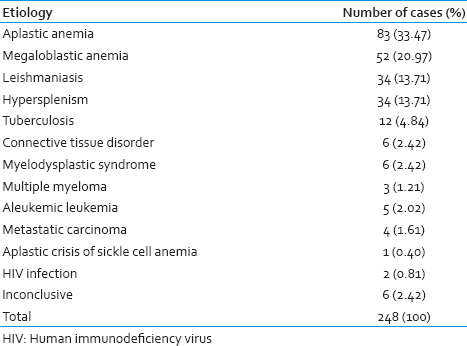
The patients have been stratified according to the age groups and the common causes of pancytopenia in Table 2. The gender distribution of different cases showed a male preponderance in the cases of aplastic anemia, megaloblastic anemia, leishmaniasis, and hypersplenism [Table 3]. The hematological parameters in these four subgroups of pancytopenia have been shown in Table 4.
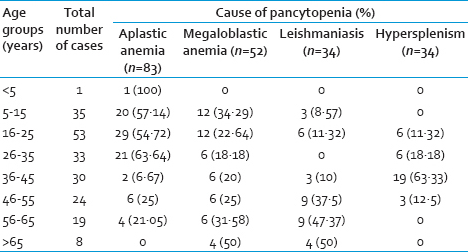

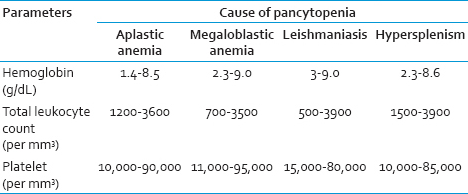
The salient physical and PBS findings of the different conditions leading to pancytopenia are summarized in Tables 5 and 6, respectively. Hepatosplenomegaly was the most common cases of megaloblastic anemia followed by leishmaniasis. In contrast, relative lymphocytosis was frequent in aplastic anemia.
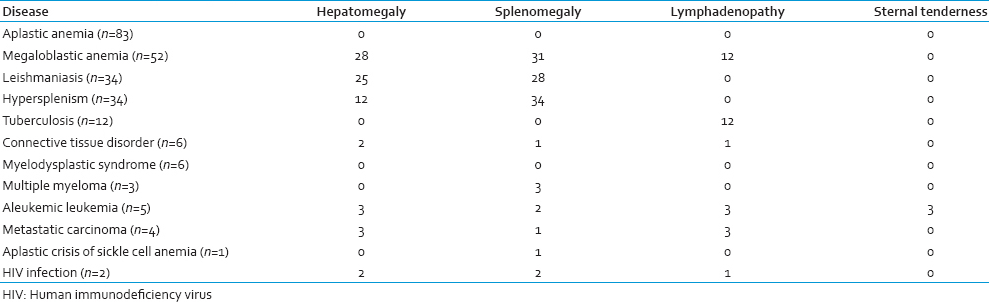
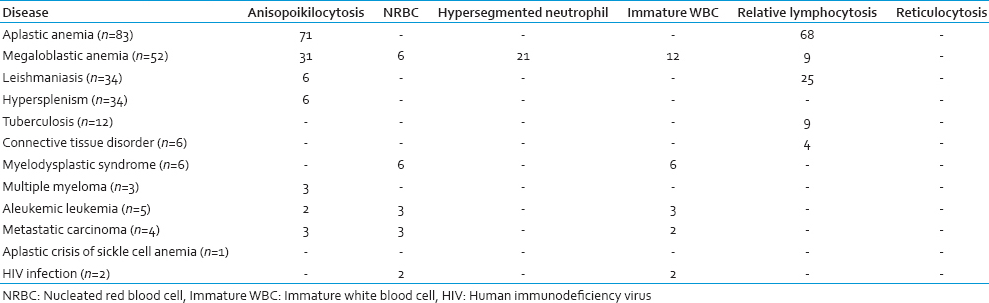
Aplastic anemia was found in 69 (83.13%) males and 14 (16.87%) females (total 83 patients; 33.47%). The age of these patients ranged from 3 to 65 years (mean age 24.31 years). All 83 patients complained of weakness, fatigue, and shortness of breath on exertion. Past history of jaundice resulting from hepatitis virus infection was present in 6 (7.23%) cases. On examination, pallor was present in all patients. Fever was detected in 37 (44.58%) patients. Forty-nine patients (59.04%) presented with bleeding manifestations. PBS examination revealed the presence of normocytic normochromic anemia. Macrocytic RBCs were found in 15 cases, and relative lymphocytosis was present in 68 cases. BMA smear examination showed hypocellular marrow with reduced erythropoiesis, myelopoiesis and megakaryopoiesis, and relative lymphocytosis was noted. BM biopsy specimens showed hypocellularity with a predominance of fat spaces and focal hematopoietic activity (hot spots). There was no significant BM dysplasia or fibrosis.
The Chi-square test was used to compare patients with aplastic anemia with those of other etiologies of pancytopenia. The patients were divided into two groups-pediatric (≤15 years) and adult (>15 years) subjects. A significant difference (P = 0.02) has been found between the two groups.
Megaloblastic anemia was diagnosed in 52 (20.97%) cases, of which 28 (53.85%) were males and 24 (46.15%) females. The mean age of these patients was found to be 29·75 years. PBS examination showed macrocytic normochromic anemia. Hypersegmented neutrophils were present in 21 cases. BM examination revealed hypercellular marrow with a reversal of myeloid to erythroid ratio. The erythroid precursors showed megaloblastic features. Giant metamyelocytes were also seen. Cobalamin (Vitamin B12) deficiency was found in 72% patients, and folate deficiency was noted in 3% patients with megaloblastic anemia. Combined cobalamin and folate deficiency were found in 2% patients.
Among the 34 (13.71%) cases of leishmaniasis diagnosed, 22 patients (64.71%)) were males and 12 (35.29%) females. Their mean age was 41.1 years. Fever was the presenting symptom in 18 (52.94%) of these patients. Serum total protein level was found to be raised in 25 patients with a mean protein level of 10.4 g/dl and an altered albumin: Globulin value in 19 cases. PBS revealed normocytic normochromic anemia. BM examination showed both intracellular and extracellular amastigote forms of Leishmania donovani. The increase in plasma cells in the marrow was also noted in 24 cases.
Twenty-five (73.53%) males and 9 (26.47%) females were diagnosed (total 34 patients; 13.71%) having hypersplenism. The age of these patients ranged from 23 to 50 years with a mean age of 36.8 years. Twelve (35.29%) patients provided a history of portal hypertension. Generalized weakness, pallor, and splenomegaly were present in all of these patients. Twenty-two of them had a fever. Normocytic normochromic anemia was found in the PBS of the patients. BM examination was unremarkable with the presence of normocellular or hypercellular marrow.
Tuberculosis accounted for 12 (4.84%) cases who presented with weakness, weight loss, prolonged low-grade fever, and pallor. Their BM examination was undertaken to rule out a hematological malignancy. BM smears of these patients revealed the presence of lymphoplasmacytic infiltration without any evidence of hypocellularity or neoplastic changes. Subsequently Mantoux test was done, which yielded a positive result. A diagnosis of tuberculosis was thus rendered in these pancytopenic patients by corroborating with clinical history and findings of other relevant investigations. Six patients (2.42%) included in our study were diagnosed cases of RA. Their BM examination revealed small aggregates of lymphocytes and plasma cells.
Six (2.42%) cases of Myelodysplastic syndrome (MDS) were diagnosed in the present study, having a mean age of 42 years. On examination, all 6 patients were found to have pallor. PBS examination showed macrocytic normochromic anemia, nucleated red blood cells, and immature white blood cells. BM examination revealed various dysplastic changes affecting different cell lineages. Megaloblastic changes and nuclear budding were seen in the erythroid series. In one case, increased blast cells were present (11%). Micromegakaryocytes were also noted. Serum Vitamin B12, folic acid, and ferritin levels were normal in these patients.
Three (1.21%) cases of multiple myeloma (MM), 5 (2.02%) cases of aleukemic leukemia, 4 (1.61%) cases of metastatic carcinoma, 1 (0.40%) case of aplastic crisis of sickle cell anemia, and 2 (0.81%) cases of Human immunodeficiency virus (HIV) infection were identified. However, in 6 (2.42%) patients with pancytopenia the results of the investigations undertaken in this study remained inconclusive, and the underlying condition could not be diagnosed.
DISCUSSION
Pancytopenia is not a disease entity per se; rather it is a manifestation of numerous disease processes that lead to the simultaneous presence of anemia, leukopenia, and thrombocytopenia in the peripheral blood of an individual. Despite being a common hematological problem, a thorough search of the literature revealed only a few studies that attempt to delineate the causes of pancytopenia.[5] Since geographical distribution is one of the important factors that influence the cause of pancytopenia, studies from various regions of different countries can be useful for understanding the underlying disease processes that lead to this manifestation, which in turn would help define the required diagnostic modalities and therapeutic approaches.
In the present study, the male to female ratio was found to be 1.7:1. The mean age of our patients was 33 (SD ± 18 years). In a similar study conducted by Das Makheja et al., 62 patients with pancytopenia were included and their average age was 37.76 years.[2] The male: female ratio in their study was 1.38:1.
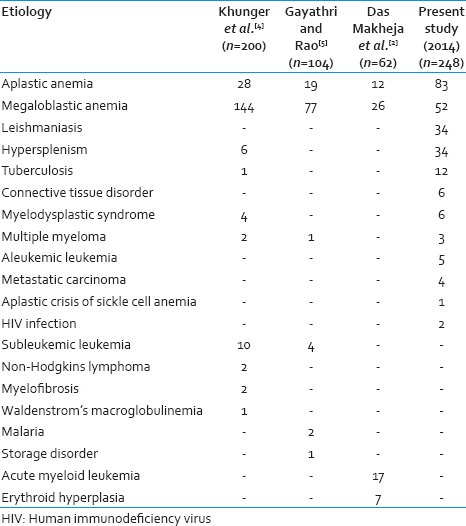
The most common cause of pancytopenia was aplastic anemia (33.47%) followed by megaloblastic anemia (20.97%). Kumar et al., found comparable results in their study: 31.82% patients were diagnosed with the aplastic anemia while megaloblastic anemia was found in 24.03% cases.[10] However in other Indian studies, megaloblastic anemia has been documented as the most common cause of pancytopenia.[45] A comparison of various causes of pancytopenia outlined in different studies has been shown in Table 7.
In the present study, aplastic anemia was also the most common cause of pancytopenia in the pediatric age group (3–15 years). Zeb Jan et al., studied the causes of pancytopenia in children aged between 6 months and 14 years and found aplastic anemia to be the most common disease responsible.[11] The Chi-square test revealed a significant difference (P = 0.02) between the number of pediatric (≤15 years) and adult (>15 years) patients with aplastic anemia, when compared with those of other etiologies of pancytopenia. This showed that compared to other etiologies, aplastic anemia is more commonly responsible for pancytopenia in the pediatric population. It must be borne in mind that in our part of the world, when an adult patient presents with pancytopenia, causes other than aplastic anemia must be initially suspected, whereas aplastic anemia must be first ruled out in a pancytopenic child.
Almost all the patients included in our study complained of weakness. On examination, anemia was noted in all the patients. Splenomegaly was another common finding that we encountered, being present in 47.98% cases (119 out of 248 patients) in our study. We reported hepatomegaly in 36.69% cases (91 out of 248 patients). Khunger et al., reported in their study that pallor was present in 100% patients, splenomegaly in 32.5% patients and hepatomegaly also in 32.5% patients.[4]
PBS findings of the present study were comparable to those mentioned in similar studies undertaken previously. Relative lymphocytosis was noted in 81.93% cases of aplastic anemia in the present study. Khunger et al. reported relative lymphocytosis in 85.71% cases of aplastic anemia, whereas Gayathri and Rao mentioned its presence in 52.63% aplastic anemia cases.[45] In this study, hypersegmented neutrophils were present in 40.38% cases of megaloblastic anemia. Hypersegmented neutrophils were seen in 51.35% cases of megaloblastic anemia by Gayathri and Rao.[5]
Premkumar et al. stated that in contrast to developed countries where pancytopenia is mostly attributed to malignancy or marrow aplasia, infectious diseases such as tuberculosis, leishmaniasis, and HIV constitute important etiologies of pancytopenia in countries like India.[12] Leishmaniasis constituted 13.58% of our cases, and 7.41% cases were those of tuberculosis and other connective tissue diseases. HIV infection was responsible for pancytopenia in 1.21% cases in this study.
MDS constituted 2.42% of total cases in our study compared to Khunger et al., who reported an incidence of 2% of MDS in New Delhi.[4]
Three cases (1.21%) of MM, 5 (2.02%) cases of aleukemic leukemia, 4 (1.61%) cases of metastatic carcinoma, 1 (0.40%) case of aplastic crisis of sickle cell anemia, and 2 (0.81%) cases of HIV infection were found in the present study. Gayathri and Rao. reported an incidence of 0.96% of MM in their series of 104 patients with pancytopenia.[5] However, a higher incidence (4%) of MM was reported by Khodke et al.[13] In contrast to our findings, Kumar et al., noted a 12% incidence of aleukemic leukemia in their study.[10] Some other causes of pancytopenia reported in various studies include malaria, Hemophagocytic syndrome, Waldenstrom's macroglobulinemia, Myelofibrosis, and Niemann-pick disease.[345]
Vitamin B12 deficiency was found to be more common than folate deficiency in patients with megaloblastic anemia, in the present study. This is consistent with the results of similar studies conducted in India and its neighboring countries.[1214]
A conclusive diagnosis was achieved in 97.58% cases of pancytopenia by means of thorough history taking, clinical examination, biochemical studies, PBS, and BM examinations. Jha et al., found that they could diagnose 77% of pancytopenia cases by BM examination.[15] Authors of the present study are in agreement with Das Makheja et al., who concluded in their study that BM examination is the single most useful investigation for the diagnosis of underlying cause in pancytopenia patients.[2]
Pancytopenia is a fairly common hematological problem. However, there is not much data available, outlining the underlying condition causing pancytopenia. Tilak and Jain., had attributed the variations in the frequency of various etiologies causing pancytopenia to numerous factors such as differences in diagnostic criteria, study period, laboratory investigations used, geographic area, genetic differences in the study population, and varying exposure to cytotoxic agents.[3]
Therefore, it is concluded that similar studies with large sample sizes from various regions of the country must be encouraged. This will significantly help to establish appropriate clinical faculties for the management of critical diseases such as aplastic anemia and hematological malignancies. Such studies will not only help in the better understanding of the underlying disease processes leading to pancytopenia but also contribute toward the better management of such patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Pancytopenia; aplastic anemia. In: Saxena R, Pati HP, Mahapatra M, eds. de Gruchy's Clinical Hematology in Medical Practice (6th Adapted Edition). Greater Noida: Wiley India Pvt. Ltd; 2013. p. :106-19.
- [Google Scholar]
- The common causes leading to pancytopenia in patients presenting to tertiary care hospital. Pak J Med Sci. 2013;29:1108-11.
- [Google Scholar]
- Pancytopenia – A clinico-hematologic analysis of 77 cases. Indian J Pathol Microbiol. 1999;42:399-404.
- [Google Scholar]
- Pancytopenia – A clinico haematological study of 200 cases. Indian J Pathol Microbiol. 2002;45:375-9.
- [Google Scholar]
- An etiological reappraisal of pancytopenia – Largest series reported to date from a single tertiary care teaching hospital. BMC Hematol. 2013;13:10.
- [Google Scholar]
- Evaluation of bone marrow in patients with pancytopenia. J Nepal Med Assoc. 2012;2:265-71.
- [Google Scholar]
- Diagnoses in patients with severe pancytopenia suspected of having aplastic anemia. Eur J Haematol. 1990;45:11-4.
- [Google Scholar]
- Pancytopenia in children: A 6-year spectrum of patients admitted to Pediatric Department of Rehman Medical Institute, Peshawar. Pak J Med Sci. 2013;29:1153-7.
- [Google Scholar]
- Cobalamin and folic Acid status in relation to the etiopathogenesis of pancytopenia in adults at a tertiary care centre in north India. Anemia. 2012;2012:707402.
- [Google Scholar]
- Vitamin B12 deficiency – A major cause of megaloblastic anaemia in patients attending a tertiary care hospital. J Ayub Med Coll Abbottabad. 2009;21:92-4.
- [Google Scholar]
- Bone marrow examination in cases of pancytopenia. JNMA J Nepal Med Assoc. 2008;47:12-7.
- [Google Scholar]





