Translate this page into:
Evaluation of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide method for assessing biofilm formation in vitro by Trichosporon spp.
Address for correspondence: Dr. Thayanidhi Premamalini, Department of Microbiology, Ramachandra Institute of Higher Education and Research, Chennai - 600 116, Tamil Nadu, India. E-mail: drtpremamalini@gmail.com
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
BACKGROUND:
Invasive infections due to Trichosporon spp. have increased recently and are frequently associated with indwelling medical devices. Such infections which are associated with biofilm formation do not respond to the routinely used antifungal agents and are often persistent, associated with high mortality rate. Various methods have been described by researchers to evaluate and quantify the biofilm formation.
AIM:
This study was conducted to compare two methods of biofilm production by Trichosporon sp, i.e., test tube method with crystal violet (CV) staining and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
MATERIALS AND METHODS:
Seventy-two clinical isolates of Trichosporon spp. collected from various sources were considered for the study. The identity of all the isolates was genotypically confirmed by Trichosporon-specific polymerase chain reaction (PCR). The isolates were further speciated phenotypically using biochemical profile and growth characteristics which identified the isolates as Trichosporon asahii (64/72), Trichosporon asteroides (5/72), Trichosporon cutaneum (2/72), and Trichosporon mucoides (1/72). Biofilm production was then evaluated and compared by test tube-CV method and MTT assay.
RESULTS:
All the Trichosporon isolates produced biofilm by MTT assay, whereas only 42 (53.6%) of the isolates were detected to be biofilm producers by CV method. Furthermore, MTT assay could differentiate better between weak and moderate biofilm producers as compared to CV method.
CONCLUSION:
Hence, MTT assay is a reliable method for quantification of biofilm produced by Trichosporon spp. using 96-well microtiter plate.
Keywords
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
biofilm
crystal violet method
Trichosporon sp
Introduction
Biofilms are microbially derived sessile community of cells that are irreversibly attached either to the surface or each other.[1] They can also form in other environments such as liquid-air interfaces. The properties of this group of adherent cells are distinct from those of free-floating (planktonic) cells.[2] An important characteristic of biofilms, compared to the planktonic cells, is the greater resistance of these cells to chemical and physical insults.[3]
The genus Trichosporon is an anamorphic basidiomycetous yeast, with a distinct morphological characteristic feature of budding yeast cells and true mycelium which disarticulates to form arthroconidia. Disseminated life-threatening infections due to Trichosporon spp. is common in patients with underlying hematological malignancies, extensive burns, solid tumors and transplant recipients, which accounts for 10% of all confirmed cases of disseminated fungal infections. Such infections due to invasive Trichosporon spp. are usually associated with central venous catheters, vesical catheters, and peritoneal catheter-related devices.[4] Prosthetic devices could act as substrates for adhesion and promote the growth of biofilms.[5] The ability of the Trichosporon spp. to adhere to and form biofilms on implanted devices can account for the progress of invasive trichosporonosis. The formation and expression of biofilms by Trichosporon spp. are frequently associated with increased antifungal resistance due to upregulated drug efflux and other factors.[6] This can promote the escape from host immune responses and antifungal drugs.[4] At times regardless of the use of antifungal drugs to treat trichosporonosis, the infection is often persistently associated with high mortality. Since these biofilms are largely resistant to the current antifungal agents, high antifungal doses together with the removal of the colonized medical device are generally required to treat the infection. However, this may be dangerous or create complications in severely ill patients.[7]
Hence, this study was undertaken to assess the formation of biofilm in-vitro by Trichosporon spp isolated from clinical specimens. The purpose of this study was 2-fold. The first objective was to evaluate biofilm formation by MTT assay. The second objective was to compare the MTT assay done in microtiter plate with the standard CV method performed in test tube.
Materials and Methods
Seventy-two strains of Trichosporon species isolated from clinical samples were used for the study.
Type of study: Hospital-based descriptive study.
Period of study: January 2011 to July 2016.
Place of study: Sri Ramachandra Medical College and RI, Chennai, Tamil Nadu, India.
Collection of isolates
Yeast-like colonies from clinical samples were initially observed for microscopic characteristics by Gram staining, Dalmau technique, and urease production. The isolates which showed the budding yeast cells, hyphae and arthroconidia by microscopy and were urease positive, were provisionally identified as Trichosporon species. These isolates were preserved at −20°C on skimmed milk medium until use.[8] Majority of our isolates were from urine, i.e., 43 (59.7%), 12 (16.7%) isolates were from blood, 7 (9.7%) Trichosporon isolates grew from samples collected by percutaneous nephrostomy. The other isolates recovered from samples sources such as respiratory, pus and peritoneal dialysis fluid and their distribution is shown in Figure 1.
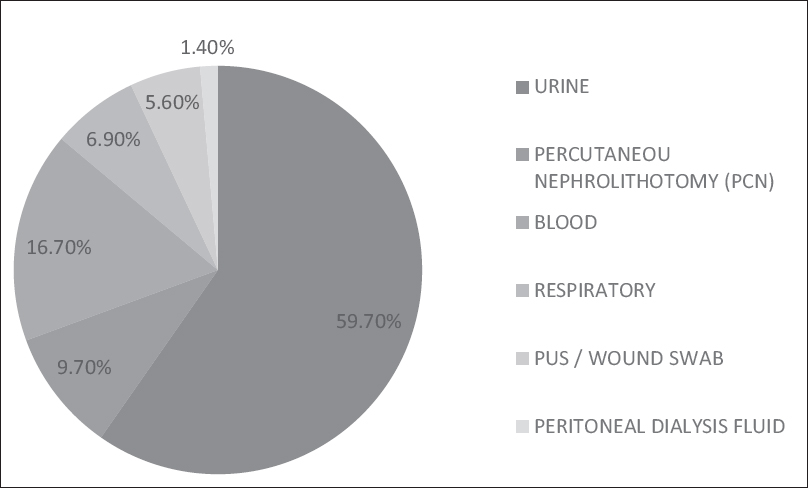
- Sample source distribution of Trichosporon spp.
Control strains
For phenotypic characterization and Trichosporon-specific polymerase chain reaction
Trichosporon asahii MTCC 6179, Trichosporon asteroides MTCC 7632, Trichosporon cutaneum var. cutaneum MTCC 1963, Trichosporon jirovecii MTCC 9036, Candida albicans ATCC 90028, Candida krusei ATCC 6258.
For biofilm production
T. asahii MTCC 6179, T. asteroides MTCC 7632, T. cutaneum var. cutaneum MTCC 1963 and T. jirovecii MTCC 9036, Candida krusei ATCC 6258, and Candida tropicalis ATCC 750.
Trichosporon specific polymerase chain reaction
DNA extraction
Genomic DNA from clinical isolates, reference strains of Trichosporon species and reference strains of Candida species were isolated by in-house method.[9] Briefly, Briefly, 400 μl of lysis buffer (10mM TRIS, pH - 8), 1 mM ethylene diamine tetra acetic acid EDTA (pH - 8), 3% Sodium dodecyl sulfate (SDS) and 100 mM NaCl was taken in a 1.5-ml centrifuge tube. A loop full of Trichosporon culture was suspended in the lysis buffer and heated at 100°C in water bath for 1 min. An equal volume of Phenol: Chloroform was added to this suspension and mixed well. It was then centrifuged at 10,000 rpm for 10 min. The aqueous layer was transferred to a fresh centrifuge tube, and the step was repeated again by adding chloroform to the supernatant. The DNA was precipitated with cold isopropyl alcohol, centrifuged and washed with 70% ethanol and dried. The pellet was later re-suspended in 30 μl of Tris-EDTA buffer and stored at 20°C until use.
Trichosporon-specific polymerase chain reaction
Trichosporon genus-specific primers (TRF-5’AGAGCCTACCATGGTATCA 3’ TRR- 5’TAAGACCCAATAGAGCCCTA 3’) would precisely amplify only Trichosporon species, by aligning with the small subunit (SSU) of ribosomal DNA (rDNA) sequences, since this region is not conserved in other medically important yeasts.[10] The polymerase chain reaction (PCR) master mix was prepared containing 25 μl of PCR mix (Takara, Japan), 1 μl of forward (TRF) and reverse primer (TRR) (GeNei, Bengaluru), 1 μl of template DNA, and the volume made up to 50 μl with sterile nuclease-free water. The reaction mixtures were amplified in a thermal cycler (Veriti 96 well, Applied Biosystems, USA), with the following program: 95°C for 7 min, followed by 30 cycles consisting of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s, with a final extension period at 72°C for 10 min.[10] After thermal cycling, 10 μl of the amplified product was run on a 1.5% (wt/vol) agarose gel, stained with ethidium bromide, and visualized with ultraviolet light.
Species level phenotypic identification
Microscopic characteristics of the isolates were studied using Dalmau technique on cornmeal agar with 1% tween 80. The plates were incubated in a moist chamber at 25°C for 3–5 days and observed under high power magnification for morphological features such as hyphae, budding yeast cells, arthroconidia, appressoria, and sarcinae. Biochemical characterization was done as described by De Hoogs et al., 2000.[11] The isolates were initially tested for fermentation and assimilation of basic sugars such as glucose, maltose, sucrose, lactose, galactose, and trehalose. Later, they were identified till species level, by physiological tests such as fermentation and assimilation of and growth on carbon sources, growth at various temperatures and 0.1% cycloheximide.[11]
Test for biofilm formation
Biofilm formation – Test tube method by crystal violet staining
Trichosporon isolates were tested for biofilm production by a modification of the standard method, using test tube and CV staining.[12] Two milliliters Sabouraud dextrose broth in 12 mm × 75 mm borosilicate test tubes were inoculated with a loopful of microorganisms from overnight culture plates and incubated for 48 h at 37°C, after which the contents were decanted and washed with phosphate buffered saline (PBS) (pH 7.3) and left to dry at room temperature. Then, the tubes were stained with 4% solution of CV. Each tube was then gently rotated to ensure uniform staining, and then the contents were gently decanted. The tubes were placed upside down to drain and then observed for biofilm formation.[12] All the isolates were tested in triplicates for confirmation of the findings. The formation of a visible film which lined the wall and bottom of the tubes is considered as positive. Ring formation at the liquid interface was not regarded as indicative of biofilm formation. The scoring was done by visual observation (absent – 0; weak – 1; moderate – 2; and strong – 3).[13]
Biofilm production – 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide assay
MTT assay was done in a 96-well microtiter plate for the development of fungal biofilm. This assay includes the formation of multiple equivalent fungal biofilms on the bottom of wells of microtiter plates, combined with a colorimetric method that measures the metabolic activities of cells within the biofilm based on the reduction of the dye MTT.
Biofilm formation
To evaluate biofilm formation of Trichosporon spp. isolates in a 96-well microtiter plate, we adopted the protocol of Jin et al. and Di Bonaventura et al. with modifications.[514] Clinical isolates of Trichosporon spp. and control strains were grown in yeast peptone dextrose broth at 35°C for 48 h. They were subcultured in RPMI 1640 medium adjusted to pH 7.0 with 0.165 M MOPS (3-(N-morpholino) propanesulfonic acid) overnight under agitation at 37°C. The cells were collected by centrifugation and washed twice with sterile PBS. They were re-suspended in RPMI 1640 (pH 7.0-MOPS), and the inoculum is adjusted to 0.5 Mcfarland turbidity standard which corresponds to 105 cells/ml. Cell suspension of 100 μl was added to 96-well flat-bottomed polystyrene plates. Eight wells were inoculated for single isolate. The plates were incubated at 37°C for 90 min (adhesion time). After incubation, the wells were washed twice with 150 μl of PBS to remove the nonadherent cells. Finally, 150 μl of RPMI 1640 (pH 7.0-MOPS) was added to each well. The plate was incubated at 37°C for 48 h (biofilm formation). Culture media were changed every 24 h.
Biofilm quantification-3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide assay
Sessile cells were quantified using MTT assay as per Traba and Liang and Tsang et al. with modifications.[1516] For the assay, MTT is diluted 1:5 from the stock solution, i.e., 1 μg/mL working solution of MTT was prepared by diluting 5 μg/mL stock solution. To quantify the adhered living cells, 50 μL of working solution of MTT was added to each well and incubated at 37°C for 4 h. After incubation, MTT solution was aspirated and 100 μl of dimethyl sulfoxide was added to each well to solubilize the insoluble end product formazan. The change in color in the MTT reduction assay, which represents the direct association of the metabolic activity of cells within the biofilm, was then measured spectrophotometrically in a microtiter plate reader. The absorbance was measured by setting the detection and reference wavelengths at 450/620 nm (read/reference), respectively. Each experiment was performed in triplicate, and biofilm quantifications were expressed as means of 24 readings (wells) per isolate ± standard deviation (SD).
For easy interpretation, the isolates were categorized based on OD values if OD <ODc (cut off value) – No biofilm producer; >ODc to <2ODc – Weak biofilm producer; >2ODc to <4 ODc – Moderate biofilm producer; and >4 ODc – Strong biofilm producer.[17]
Statistical analysis
All statistical analysis was performed using Statistical Package for Social Science (SPSS Inc, version 17, Chicago) for Microsoft Windows. Descriptive statistics were presented as numbers and percentages; the data were expressed as Mean and SD A Chi-squared test was used for comparison between two attributes. A two-sided P < 0.05 was considered statistically significant.
Results
All the 72 clinical isolates considered for our study and the four reference strains exhibited morphological and microscopic characteristics compatible with Trichosporon genus. PCR was performed with Trichosporon genus-specific primers to double check for accurate identification of the genus. This pair of primer is Trichosporon specific and amplifies part of the nucleotide sequences of the rDNA SSU (18S). DNA bands of approximately 170 bp were obtained for all the isolates tested and for Trichosporon reference strains [Figure 2]. In addition, there was no amplification of DNA isolated from Candida spp. (negative control). Therefore, all our strains were confirmed to belong to the genus Trichosporon.
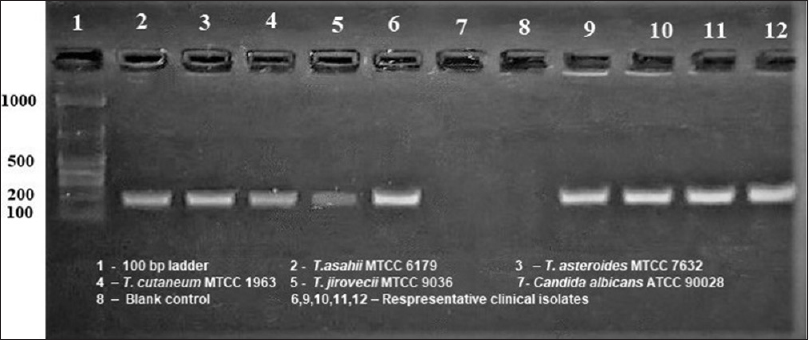
-
Trichosporon specific polymerase chain reaction
The isolates were further speciated based on phenotypic characteristics. Fermentation was absent in all the isolates. Different biochemical and growth profiles of Trichosporon spp. are shown in Table 1. Hence, the isolates were identified phenotypically as T. asahii (64/72), T. asteroides (5/72), T. cutaneum (2/72), and Trichosporon mucoides (1/72).

Biofilm production
Out of the 72 Trichosporon isolates studied, only 42 (58.3%) of them produced biofilm by test tube (CV) method and all the isolates produced biofilm by MTT assay.
Biofilm quantification by crystal violet method
Of the 42 biofilm producers detected by CV method, 5 (6.9%) were weak biofilm producers, 17 (23.6%) were moderate biofilm producers, and 20 (27.8%) were strong biofilm producers.
Biofilm quantification by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
All the 72 Trichosporon isolates produced biofilm by MTT assay, out of which, 16 (22.2%) were weak biofilm producers, 29 (40.3%) were moderate biofilm producers, 27 (37.5%) were strong biofilm producers. In this assay, the mean A450/620 (read/reference) value was 0.631(SD + 0.3) with a range of 0.197–1.42 that corresponds to 7.2-fold difference between the highest and the lowest biofilm producer. Majority of the blood isolates and PCN isolates, i.e., 10/12 and 5/7 were strong biofilm producers [Table 2]. The mean absorbance value for blood and PCN isolates was found to be high, i.e., 9.5 and 9.8, respectively [Figure 3]. Although isolates from particular sample sources (peritoneal fluid, blood, and PCN) were observed to be high biofilm producers, there was no much difference observed in biofilm production among phenotypically characterized species of the genus Trichosporon.

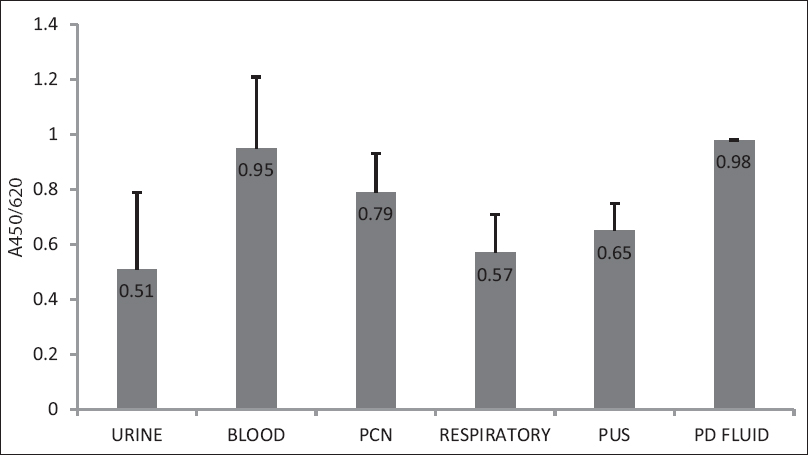
- Mean OD values of different sample sources
The OD values of the control strains used in our study are shown in Figure 4. There was a significant difference between the biofilm production by CV method and MTT assay for the reference strains [Table 3].
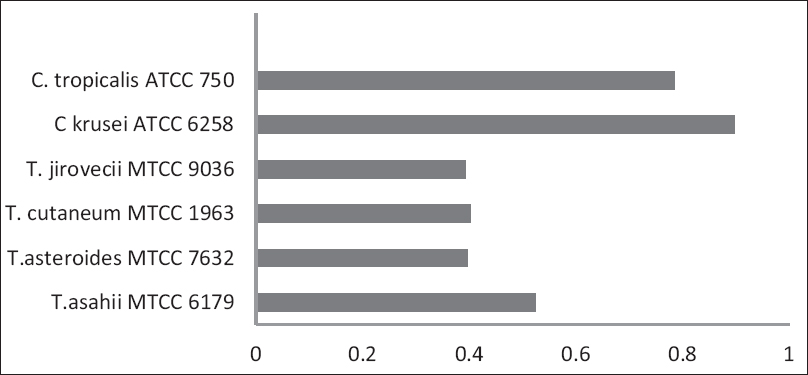
- OD values of Control strains of Trichosporon spp. and Candida sp.

Comparison of biofilm production
The MTT assay detected more weak, moderate and strong biofilm producers than CV method. The isolates which were negative by CV method were either weak (53.3%), moderate (43.3%), or strong (3.3%) biofilm producers by MTT assay [Table 4].

Discussion
Trichosporon species are emerging nosocomial pathogens, frequently associated with infections in indwelling medical devices.[1819] Their infective capacity depends on specific virulence factors, which gives them the ability to colonize mucosal or synthetic surface and invade host tissues by disrupting host-cell membranes. Hence, persistent and recurrent infections are a common occurrence in such cases.[20] Furthermore, biofilm production renders them refractory to antimicrobial treatment, therefore high antimicrobial concentrations are required to inactivate such organisms growing in a biofilm.
Various methods have been employed for the detection of fungal biofilms. Newer techniques such as DNA extraction and quantification polymerase chain reaction is time-consuming and expensive.[21] In this study, we compared two frequently described, different procedures for quantifying the biofilm formation. All the 72 clinical isolates of Trichosporon spp. were tested for biofilm production by test tube method with CV staining and MTT assay using 96-well microtiter plate.
The test tube method using CV staining identified 42/72 (58.3%) of the isolates as biofilm producers, out of which 5 (6.9%) were weak biofilm producers, 17 (23.6%) were moderate biofilm producers, and 20 (27.8%) were strong biofilm producers. CV is a reliable tool for determining bulk biofilm formation produced by fungal cells since it stains the metabolically active and inactive cells in mature biofilms.[2223]
All the isolates tested in our study produced biofilm by MTT assay, in which 16 (22.2%) were weak biofilm producers, 29 (40.3%) were moderate biofilm producers, and 27 (37.5%) were strong biofilm producers. This finding was similar to the study done by Wei sun et al., where all the isolates produced biofilm on polystyrene surface.[24] This phenomenon has important clinical implications for therapy of biofilm-associated infection, which is difficult to treat and recurs easily.[25]
Biofilm production has been evaluated in laboratory settings using microtiter plates.[26] This method is most popular due to its versatility, simplicity, reproducibility, and efficiency. MTT-reduction assay shows excellent correlation between cellular density and metabolic activity, thus providing semiquantitative measurement of biofilm formation.[2327] This colorimetric assay has the additional benefit in contrast to other methods such as CV staining that, it correlates with cell viability which is particularly useful for measuring the effects of drugs on biofilm cells. MTT is reduced by mitochondrial dehydrogenases of metabolically active biofilm cells and is, therefore, the best approach to test the effect of drug exposure on the biofilm viability.[2829]
Isolates from peritoneal fluid, blood, and PCN had high mean OD values in MTT assay, and majority of them (i.e., 1/1; 10/12; 5/7) were strong biofilm producers. In another study by Iturrieta-González et al., Trichosporon spp. were high biofilm producers in blood, urine, and skin samples.[30] High adherence and biofilm formation may promote colonization and establishment of infection even in samples collected from superficial sites.
However, test tube method by CV staining when compared to MTT assay, could not identify weak and moderate biofilm producers efficiently. The detection percentage was high in MTT assay (22.2% and 40.3%) when compared to CV assay (6.9% and 23.6%). This finding is consistent with the observation made by Mathur et al. Test tube method with CV staining can be a good technique for biofilm quantification, but it has a high degree of subjective variability in reading and cannot accurately detect moderate-to-weak biofilm producers.[13]
Conclusion
MTT assay using microtiter plates detects more biofilm producers compared to test tube method by CV staining, in our study. This method has been widely used by researchers to study biofilm formation and quantification, making it more reliable and comparable among different laboratories, a necessary step toward the standardization of antifungal susceptibility testing of biofilms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167-93.
- [Google Scholar]
- Biofilms in lab and nature: A molecular geneticist's voyage to microbial ecology. Int Microbiol. 2010;13:1-7.
- [Google Scholar]
- Biofilm formation by the emerging fungal pathogen Trichosporon asahii: Development, architecture, and antifungal resistance. Antimicrob Agents Chemother. 2006;50:3269-76.
- [Google Scholar]
- Candida biofilms: Antifungal resistance and emerging therapeutic options. Curr Opin Investig Drugs. 2004;5:186-97.
- [Google Scholar]
- Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1-45.
- [Google Scholar]
- Morphological and biochemical characterization of the aetiological agents of white piedra. Mem Inst Oswaldo Cruz. 2008;103:786-90.
- [Google Scholar]
- Molecular species identification of Candida from blood samples of Intensive Care Unit patients by polymerase chain reaction-restricted fragment length polymorphism. J Lab Physicians. 2012;4:1-4.
- [Google Scholar]
- Rapid detection of species of the opportunistic yeast Trichosporon by PCR. J Clin Microbiol. 1998;36:1458-60.
- [Google Scholar]
- Atlas of clinical fungi (2nd ed). Guanabara, Rio de Janeiro, Brazil: Centraalbureauvoor Schimmelcultures Utrecht, The Netherlands, and Universitat Rovirai Virgili, Reus, Italy; 2000.
- Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318-26.
- [Google Scholar]
- Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 2006;24:25-9.
- [Google Scholar]
- Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789-98.
- [Google Scholar]
- Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling. 2011;27:763-72.
- [Google Scholar]
- Purpurin suppresses Candida albicans biofilm formation and hyphal development. PLoS One. 2012;7:e50866.
- [Google Scholar]
- Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891-9.
- [Google Scholar]
- Trichosporon species infection in bone marrow transplanted patients. Diagn Microbiol Infect Dis. 2001;39:161-4.
- [Google Scholar]
- Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48-66.
- [Google Scholar]
- Comparative analysis of Candida biofilm quantitation assays. Med Mycol. 2012;50:214-8.
- [Google Scholar]
- Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475-9.
- [Google Scholar]
- Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450-2.
- [Google Scholar]
- Trichosporon asahii causing nosocomial urinary tract infections in intensive care unit patients: Genotypes, virulence factors and antifungal susceptibility testing. J Med Microbiol. 2012;61:1750-7.
- [Google Scholar]
- Biofilm production by isolates of candida species recovered from nonneutropenic patients: Comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol. 2002;40:1244-8.
- [Google Scholar]
- Optimizing a candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J Clin Microbiol. 2011;49:1426-33.
- [Google Scholar]
- The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia. 2005;159:353-60.
- [Google Scholar]
- Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8:442-50.
- [Google Scholar]
- Multiple species of Trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS One. 2014;9:e109553.
- [Google Scholar]





