Translate this page into:
Evaluation of Endometrium for Chronic Endometritis by Using Syndecan-1 in Abnormal Uterine Bleeding
Address for correspondence: Dr. K. Vidyavathi, E-mail: vidyaraj74@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Chronic endometritis is a condition observed in 3-10% of women with abnormal uterine bleeding (AUB). Diagnosis depends upon the histological detection of plasma cells within the inflammatory infiltrate in the endometrium. Plasma cells on H and E may be obscured by a mononuclear infiltrate, plasmacytoid stromal cells, abundant stromal mitosis, a pronounced predecidual reaction, menstrual features or secondary changes due to exogenous progesterone treatment prior to biopsy.
Aims:
The objective of this study was to determine utility of syndecan-1 in diagnosis of chronic endometritis in patients with AUB, and to see if any of the secondary histologic features in endometrial biopsy, correlated with the presence of plasma cells on immunohistochemistry (IHC).
Materials and Methods:
Fifty endometrial biopsies with a clinical diagnosis of AUB were taken. Endometrium in proliferative phase, secretory phase, endometrial polyps, and disordered proliferative endometrium were studied for the presence of plasma cells. IHC was done using syndecan-1. The secondary histologic features of chronic endometritis like gland architectural irregularity, spindled stroma, stromal edema and hemorrhage with the presence of plasma cells was statistically analysed. Values of P < 0.05 were considered as significant.
Results:
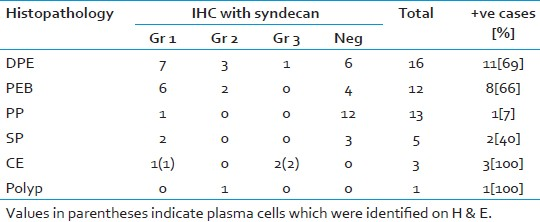
Plasma cells were seen in 11 (69%) of DPE, 8 (66%) of PEB, and 1 (7%) of normal proliferative endometrium and in 2 (40%) of secretory endometrium. Presence of stromal breakdown showed a significant association with plasma cells (P = 0.02) whereas gland architecture irregularity (P = 0.28), stromal edema (P = 0.71) and spindled stromal (P = 0.72) did not show a significant association.
Conclusions:
Plasma cells were significantly present in AUB patients. Syndecan-1 maybe helpful in unusual cases, where chronic endometritis is suspected as the cause of clinically significant ongoing abnormal bleeding.
Keywords
Abnormal uterine bleeding
chronic endometritis
syndecan-1
INTRODUCTION
Chronic endometritis (CE) is a persistent inflammation of the endometrium and is observed in 3-10% of women who undergo endometrial biopsy for abnormal uterine bleeding (AUB).[1] Histologically, the diagnosis of chronic endometritis is based on the presence of plasma cells in the endometrium.[1] Other associated morphological features include superficial stromal edema, increased stromal density, stromal breakdown, gland architectural irregularity, a stromal infiltrate including lymphocytes and leucocytes with a glandular leucocytic infiltrate.[2] Plasma cells have a characteristic clock face chromatin, with an eccentric nucleus and a visible perinuclear halo. However, many conditions may mimic or interfere with the search for of plasma cells on routine H and E. Plasma cells may be obscured on H and E by a mononuclear infiltrate, plasmacytoid stromal cells, abundant stromal mitosis, pronounced predecidual reaction, menstrual features or secondary changes due to exogenous progesterone treatment prior to biopsy.[134]
Ancillary diagnostic techniques have been used to identify plasma cells, like methyl green pyronin stain, immunohistochemistry (IHC) for immunoglobulin G, or syndecan and insitu hybridization for κ and λ light chains.[356] Syndecan-1 is a cell surface proteoglycan that is expressed on plasma cells (benign and malignant) and on keratinocytes, but it is not expressed by mononuclear cells, lymphocytes or endometrial stromal cells.[1]
Various studies for immunohistochemical characterization of leucocytes in endometrium have shown a statistical significant increase in T cell, B cell and macrophage numbers in acute and chronic endometritis when compared to normal endometrial tissue.[78] Presence of eosinophils has also been shown to have a significant association with CE.[9] But however, the presence of plasma cells is still considered to be the diagnostic criteria for CE.
The clinical presentation of chronic endometritis is very varied and nonspecific.[2] The patients are usually asymptomatic or present only with subtle abnormalities such as abnormal uterine bleeding, pelvic pain, dyspareunia, and leucorrhea. Various studies have disclosed the association between CE and infertility. CE is identified in 30% of patients with repeated implantation failure, 28% of the patients with unexplained infertility and 12% of patients with unexplained recurrent miscarriages.[10–12] However very few studies have been done to see the association of CE in AUB. Chronic endometritis respond to antibiotic regime or may be self-limiting as the act of curettage itself is self-therapeutic. Thus an accurate assessment of endometrial samples in abnormal uterine bleeding is necessary both as an academic interest as well as in order to avoid unnecessary surgeries.
The objective of this study was to determine the utility of syndecan-1 in aiding the diagnosis of chronic endometritis in patients with AUB, and to see if any of the secondary histologic features in endometrial biopsy, correlated with the presence of plasma cells on IHC.
MATERIALS AND METHODS
Fifty endometrial biopsies from patients with a clinical diagnosis of AUB were taken. Endometrial biopsies were subjected to routine histopathological processing and H and E stain. Endometrium in proliferative phase, secretory phase, endometrial polyps, disordered proliferative endometrium with stromal breakdown/anovulatory pattern were studied for the presence of plasma cells. Proliferative endometrium was further divide into proliferative endometrium with breakdown (PEB) and normal/inactive proliferative endometrium (PP). DPE was used to describe biopsies with irregulary spaced and dilated glands often accompanied with stromal breakdown. Stromal breakdown included presence of clustered neutrophils in the stroma with stromal cell apoptosis. Stromal hemorrhage with fragmentation alone, stromal edema or a loosely packed stroma was considered insufficient for stromal breakdown. Endometrial hyperplasia and malignancy were excluded from the study.
Endometrial biopsy were subjected to IHC, were cut and mounted on 3-aminopropyl- trethoxysilane coated slides, and stained using syndecan-1 (monoclonal mouse antihuman CD 138; Clone M115, Dako). Plasma cells were identified on H and E and Syndecan-1 staining. The immunostained slides were scored for the presence of plasma cells using light microscopy, in at least 10 high power fields. The biopsy was graded as “Negative” - when no plasma cells stained with syndecan, 1+ when <5 plasma cells were present, 2+ when 5-10 plasma cells were present, 3+ when >10 plasma cells were present.
The secondary histologic features of chronic endometritis like gland architectural irregularity, spindled stroma, stromal edema and stromal hemorrhage with the presence of plasma cells was statistically analysed using SPSS software version 14 and Fischer's exact test was analysed. Values of P < 0.05 were considered as significant.
RESULTS
Fifty endometrial biopsies were reviewed for presence of plasma cells on H and E and using IHC for syndecan-1. Age of the patients varied from 19-55 years with a median age of 40 years. Histopathology showed 16 cases of disordered proliferative endometrium, 12 cases of PEB, 13 cases of proliferative phases, five cases of secretory phase, three cases of CE and one case of endometrial polyp diagnosed on histopathology.
Syndecan- 1 showed membrane staining for plasma cells and also stained the endometrial glands [Figure 1]. IHC proved useful and increased the detection rate of plasma cells which were not identified on original H and E in biopsy showing spindled stroma, stromal breakdown and/or stromal edema [Figures 2 and 3]. Out of 50 cases, only three cases showed plasma cells on H and E, whereas 26 cases showed plasma cells on IHC. The three cases where plasma cells were identified on H and E are shown in parentheses in Table 1. On IHC, plasma cells were seen in 11 (69%) of DPE, 8 (66%) of PEB, and 1 (7%) of normal proliferative endometrium. Plasma cells were also seen in 2 (40%) of secretory endometrium. Three cases of chronic endometritis diagnosed on histopathology showed grade 1 ((1 case) and grade 3 (2 case) plasma cells. One case of endometrial polyp showed grade 2 plasma cells which was not seen on H and E. The incidences of stromal plasma cells with grading in each diagnostic category are listed in Table 1.

- Endometrium containing plasma cells showing membrane staining highlighted with Syndecan-1. ×400

- (a) Plasma cells are not readily seen in H and E slide with mucosal odema of stroma H and E ×100; (b) Syndecan-1 showed numerous plasma cells in stroma. ×100; (c) H and E slide showing prominent spindle cell component. ×100; (d) Plasma cells easily identified in spindle cell component. ×100
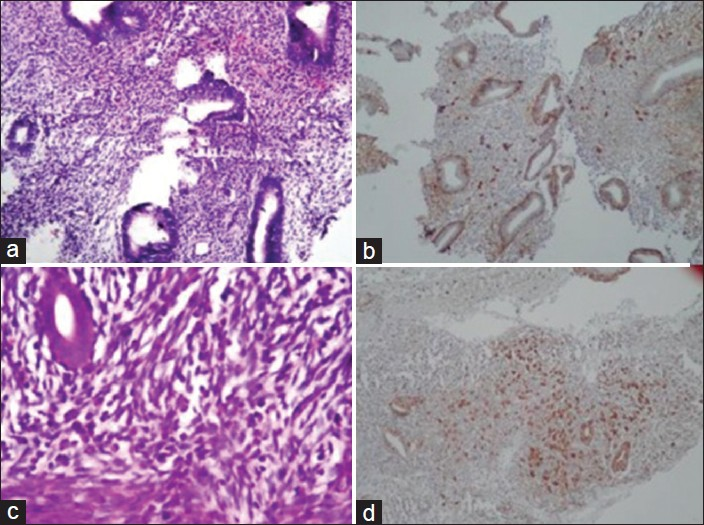
- (a) Irregular endometrial glands showing mild stromal breakdown. H and E, ×100; (b) IHC showing plasma cells concentrated beneath endometrial glands. ×100. (c). Plasma cells clearly seen on H and E, ×100; (d) Grade 3 plasma cells on IHC which are concentrated beneath glands ×100
The association of secondary histologic features like gland architecture irregularity, spindled stroma, stromal edema, and stromal breakdown with presence of plasma cells was assessed in 45 cases showing proliferative glands. Five cases of secretory phase were not included in the statistical analysis as plasma cells can be normally seen in secretory endometrium due to prominent stromal breakdown. Statistical analysis was done using SPSS software version 14. Presence of stromal breakdown showed a significant association with plasma cells (P = 0.02) whereas other histologic features like gland architecture irregularity (P = 0.28), stromal edema (P = 0.71) and spindled stromal (P = 0.72) did not show a significant association.
DISCUSSION
Chronic endometritis is considered an infectious or a reactive process. Commonly cited causes outside the post-partum period, include transvaginal infections, IUDs, submucosalleiomyomas, and endometrial polyp.[13] In other words, any cause of chronic irritation to the endometrium may result in a chronic inflammatory reaction.[13] Histopathological diagnosis is by presence of plasma cells in endometrium with or without accompanying acute inflammation and lymphocytes.[14]
Other features which can alert a pathologist are disturbances in normal growth and maturation, superficial mucosal stromal odema, stromal breakdown and characteristic spindle cell alteration of the stroma.[14] In spite of multiple histologic features, the key feature is the presence of plasma cells, diagnosis of which can be done by IHC.
Majority of DPE cases showed grade one plasma cells. PEB also showed predominantly grade one plasma cells. There was also a significant association between stromal breakdown and the presence of plasma cells on biopsy. However there was no significant association between other secondary histologic features like stromal edema, spindled stoma and gland architecture irregularity. Gilmore et al. in their study found an increased incidence of plasma cells in DPE, followed by PEB, but rare in normal proliferative endometrium.[5] This suggests that plasma cells are commonly seen in endometrium of women with focal stromal breakdown.
A higher incidence of plasma cells in proliferative endometrium was seen when compared to secretory endometrium, which was similar to the findings by Eckert LO et al.[915] However Kitaya and Yasuo found an equal incidence in proliferative phase (10.9%) and secretory phase (9.3%). This could be attributed to the type of endometrium sampled in their study. Endometrium from hysterectomy samples were studied where plasma cells were seen in the deeper endometrial stroma, whereasbiospy included only the superficial endometrial stroma.[16]
Chronic endometritis usually presents with abnormal uterine bleeding, but is often asymptomatic or may present with mild symptoms. Hence actual prevalence in population is unknown. Adegboyega PA et al., detected a 15.6% prevalence in their study which was higher than the 3%-10% reported in previous studies.[9] Smith M et al., found a 16% misdiagnosis of CE on biopsies.[13] This has been attributed to the specific immunostaining of plasma cells by Syndecan-1, which not only stains typical plasma cells, but also spindle shaped plasma cells which may be missed on a H and E. Though special stains like MGP are available and are cost effective, CD 138 or Syndecan-1 is specific for plasma cells. Using IHC as the gold standard, Miguel et al., found a sensitivity and specificity of only 75and 65% respectively on MGP.[7] This highlights the inferior performance characteristics of H and E and MGP over Syndecan. Additional factors including inexperience, fatigue, and time constraints may contribute to a missed diagnosis. Use of CD 138 immuno stain may help pathologist to reduce screening time to detect plasma cells. The increased detection rate results from IHC stained plasma cells which mimics stromal cells or lymphocytes and could not be identified with confidence on H and E.
In the present study, we found a significant 36% incidence of CE in patients presenting with AUB. Miguel et al., using flow cytometric analysis detected plasma cells among 30% of women undergoing hysterectomy for AUB.[7] This provides additional support that plasma cells should be regarded as nonspecific indicators of upper genital tract inflammation.
Various studies for IHC characterization of leucocytes in endometrium have shown a statistical significant increase in T cell, B cell and plasma cells numbers in acute and CE when compared to normal endometrial tissue.[78] Presence of eosinophils has also shown a significant association with the incidence of plasma cells.[9] However, the number of plasma cells required for diagnosis of CE is still controversial. There are currently a number of different criteria used in literature to diagnose upper genital tract inflammation. These include the presence of five or more neutrophils per high power field (HPF) and 1 or more plasma cells within endometrial stroma per low power field (LPF),[17] >2 plasma cells per HPF[18] and even the presence of a single plasma cell in the entire specimen.[19] Few authors believe that presence of one or two plasma cells with associated stromal and/or glandular changes in AUB is sufficient for the diagnosis of chronic endometritis.[79] On the other hand, if many plasma cells are present even in the absence of prominent stromal changes, a diagnosis of chronic endometritis may still be given, as the associated stromal and glandular changes are dependent on the duration of the disease, as early in the disease stromal or glandular changes may not be prominent.[9] On the contrary, Achilles et al., detected plasma cells in endometrium in a cohort of healthy asymptomatic fertile women, suggesting that plasma cells may not represent upper genital tract inflammation.[20] However, the study was limited by a small sample size and by the use og MGP stain which may not be entirely specific. One study found the presence of >1 plasma cell in endometrium to be associated with Chlamydia Trachomatis infection.[19] It is thus necessary to have a better understanding of what number of endometrial plasma cells is normal and what number of plasma cells per area of tissue correlates well with inflammation and infection. Further research into these areas is required to better define a histologic diagnosis of endometritis in the absence of other inflammatory markers such as neutrophils.
The presence of plasma cells may suggest the need for microbiological investigation to rule out any specific etiology for chronic endometritis, especially C. Trachomatis.[1921] If no infectious etiology is identified, then reactive cause for chronic irritation should be considered. In a series of women, older than 50 years, Crum and Lee attributed 36% of CE to organic or structural causes [fibroid, polyp, IUD]. Empiric treatment including antimicrobial therapy and hormonal manipulation is usually administered. Though 80% of CE is self-limiting and DandC itself is therapeutic, failure to diagnose and treat CE may result in persistent abnormal uterine bleeding that does not respond to hormonal treatment alone. Few studies have emphasised the role of empiric antimicrobial therapy in chronic endometritis and its importance in preventing morbidity of an operative procedure such as hysterectomy.[5]
The association between plasma cells and AUB still remains to be established. Kitaya et al., demonstrated that endometrium with CE uniquely expresses the chemokines CXCL1, CXCL13 and adhesion molecules select in E, implicating that local B lymphocytes are recruited from endometrial microcirculation and differentiate in situ into plasmacytes.[11] Such unusual leucocyte composition in CE may disrupt the integrity of the epithelial lining and cause endometrial shedding resulting in AUB. In the present study, CE was significantly detected in DPE. This could probably due to the effect of unopposed estrogen in the endometrium which predisposes to an inflammatory milieu by the production of cytokines and growth factors.[22]
We believe that the presence of plasma cells in AUB patients suggests that these cells should be regarded as indicators of upper genital tract inflammation. Immunohistochemistry could help pathologists save time searching for plasma cells. In conclusion, it is noteworthy to mention that use of Syndecan-1 for plasma cells may be helpful in unusual cases where chronic endometritis is suspected as the cause of clinically significant on-going abnormal bleeding.
ACKNOWLEDGEMENT
The authors thank Sri Devaraj Urs Academy of Higher Education and Research, Deemed to be University for financial support. The paper obtained from research project SDUU/02/10.
Source of Support: Financial support from Sri Devaraj Urs Academy of Higher Education and Research, Kolar.
Conflict of Interest: None declared.
REFERENCES
- Routine Syndecan-1 immunohistochemistry aids in the diagnosis of chronic endometritis. Arch Pathol Lab Med. 2004;128:1000-3.
- [Google Scholar]
- Chronic endometritis : Morphologic and clinical observations. Obstet Gynecol. 1981;58:176-84.
- [Google Scholar]
- Chronic endometritis : The role of immunohistochemistry in the detection of plasma cells. Am J Obstet Gynecol. 1983;147:812-5.
- [Google Scholar]
- Plasma cell in chronic endometritisare easily identified when stained with Syndecan-1. Mod Pathol. 2001;14:877-9.
- [Google Scholar]
- Diagnosis of chronic endometritis in biopsies with stromal breakdown. Hum Pathol. 2007;38:581-4.
- [Google Scholar]
- Detection of Kappa and lamba expressing cells in the endometrium by in situ hybridization. Int J Gynecol Pathol. 2002;21:383-90.
- [Google Scholar]
- Limitations of the criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease research. Pathol Res Pract. 2011;207:680-5.
- [Google Scholar]
- Immunohistochemical characterisation of leukocytic subpopulations in chronic endometritis. Infect Dis Obstet Gynecol. 1996;4:287-93.
- [Google Scholar]
- Relationship between eosinophils and chronic endometritis. Hum Pathol. 2010;41:33-7.
- [Google Scholar]
- Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilisation. Fertil Steril. 2010;93:437-41.
- [Google Scholar]
- Aberrant expression of selectin E, CXCL1 and CXCL13 in chronic endometritis. Mod Pathol. 2010;23:1136-46.
- [Google Scholar]
- Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011;95:1156-8.
- [Google Scholar]
- Chronic endometritis : A combined Histopathologic and clinical Review of cases from 2002 to 2007. Int J Gynecol Pathol. 2009;29:44-50.
- [Google Scholar]
- Evaluation of cyclic endometrium and benign endometrial disorders. Diagnostic Gynaecologic and Obstetric Pathology 2006:441-91.
- [Google Scholar]
- Endometritis : The clinico-pathologic syndrome. Am J Obstet Gynecol. 2002;186:690-5.
- [Google Scholar]
- Immunohistochemical and clinicopathological characterisation of chronic endometritis. Am J Reprod Immunol. 2011;66:410-5.
- [Google Scholar]
- Endometrial histopathology in patients with culture proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol. 1990;14:167-75.
- [Google Scholar]
- Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease : Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929-37.
- [Google Scholar]
- Plasma cell endometritis is associated with Chlamydia trachomatis infection. Am J Clin Pathol. 1999;112:211-5.
- [Google Scholar]
- Endometrial plasma cells : Do they indicate subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32:185-8.
- [Google Scholar]
- Chlamydia trachomatis in the endometrium : Can surgical pathologists identify plasma cells? Adv Anat Pathol. 2001;8:327-9.
- [Google Scholar]
- Inflammation and endometrial cancer : A hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14:2840-7.
- [Google Scholar]





