Translate this page into:
Evaluation of Two Phenotypic Methods for the Detection of Plasmid-Mediated AmpC β-Lactamases among Enterobacteriaceae Isolates
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
AmpC β-lactamases are cephalosporinases that confer resistance to cephalothin, cefazolin, cefoxitin, penicillin, and β-lactamase inhibitor-β-lactam combinations. Even though the AmpC resistance is reported, but the accurate occurrence of AmpC β-lactamases in Enterobacteriaceae members is still unknown. Techniques to identify AmpC producers are still evolving but not yet optimized for the clinical laboratory. Here we aimed to compare the test performance of two different phenotypic methods, that is inhibitor-based assay using boronic acid and disk approximation test for AmpC detection in Enterobacteriaceae isolates from a tertiary hospital microbiology laboratory.
Materials and Methods
The study includes 137 nonrepeat Enterobacteriaceae strains. Bacterial isolates, that yielded a zone diameter of less than 18 mm for cefoxitin by disk diffusion method were considered potential AmpC producers and further confirmed by phenotype methods—inhibitor-based assay using boronic acid and disk approximation test. A multiplex polymerase chain reaction was used to detect the most common plasmid-mediated AmpC genes: ACC, FOX, MOX, DHA, CIT, and EBC.
Results
Of the 137 clinical isolates, 58 (42.33%) were cefoxitin resistant, while 53.4 and 18.9% of the cefoxitin-resistant isolates were positive by inhibitor-based assay and disk approximation test. Multiplex PCR detected 42 (30.6%) isolates with AmpC genes. Of the 42 isolates, the inhibitor-based assay detected 25 (59.5%) isolates, while the disk approximation test detected nine (21.4%) isolates.
Conclusion
Our findings suggest that inhibitor-based assay using boronic acid can be used for the detection of the isolates that harbor AmpC β-lactamases. This method is cost-effective, simple to perform, and easy to interpret. Thus AmpC detection as a routine in clinical laboratories can help in appropriate therapeutic intervention and improved infection control.
Keywords
Enterobacteriaceae
AmpC β-lactamases
inhibitor-based assay
disk approximation test
multiplex PCR
Background
AmpC β-lactamases are cephalosporinases that confer resistance to cephalothin, cefazolin, cefoxitin, penicillin, and β-lactamase inhibitor-β-lactam combinations.[1] Under Ambler classification scheme, AmpC β-lactamases are class C enzymes, which utilize serine for beta-lactam hydrolysis.[2] AmpC β-lactamase resistance mechanisms can be: (1) inducible resistance via chromosomally encoded AmpC genes which is present in Citrobacter freundii, Enterobacter cloacae, etc., (2) non-inducible chromosomal resistance due to promoter and/or attenuator mutations seen in Escherichia coli, Shigella species, and (3) plasmid-mediated resistance in Klebsiella pneumoniae, E. coli, Salmonella species, etc.[3]
Even though the AmpC resistance is reported, but the accurate occurrence of AmpC β-lactamases in Enterobacteriaceae members is still unknown.[4] In Enterobacteriaceae, cefoxitin resistance is used for screening of AmpC β-lactamase producers. Its resistance may also be due to alterations to outer membrane permeability.[5] Techniques to identify AmpC producers are still evolving but not yet optimized for clinical laboratory.[6] Disk-based assays using cloxacillin and inhibitors (boronic acid [BA] compounds), cefoxitin-cloxacillin double disk synergy, AmpC disk tests, disk approximation tests, etc. have been developed for detection of AmpC-producing β-lactamase isolates in Enterobacteriaceae.[5,6] Molecular tests are also available but their use is restricted to research settings.[3] The Clinical and Laboratory Standards Institute (CLSI) guidelines 2019 recommended criteria for AmpC resistance detection do not exist.
However, surveillance and monitoring activity is significantly important in this epoch of multidrug resistance as failure in antimicrobial resistant mechanisms detection may result in the spread of resistant pathogens and ultimately, complicating the clinical outcome.[5] Well-designed studies on diagnostic techniques for the detection of AmpC β-lactamases, that is easy-to-perform and provides reliable results in a short time, and suitable for treatment recommendations for AmpC-producers are needed.[7]
This study aimed to compare the test performance of two different phenotypic methods that is inhibitor-based assay using BA and disk approximation test for AmpC detection in Enterobacteriaceae isolates from a tertiary hospital microbiology laboratory.
Material and Methods
Bacterial Isolate Collection and Identification
The study was based on laboratory surveillance from July 2018 to February 2019. The analysis includes 137 nonrepeat Enterobacteriaceae strains (60 E. coli, 60 K. pneumoniae, 10 Proteus species, 5 Citrobacter koseri, 1 Salmonella typhi, and 1 Enterobacter species samples). The clinical isolates were identified by their colony morphology, Gram staining characteristics, and standard biochemical tests.[8] We evaluated antimicrobial susceptibility by Kirby Bauer method as per CLSI (formerly NCCLS) guidelines, 2018.
AmpC β-lactamase Detection
Bacterial isolates that yielded a zone diameter of less than 18 mm for cefoxitin by disk diffusion method were considered potential AmpC producers[9] which was further confirmed by phenotype methods—inhibitor-based assay using BA and disk approximation test.
Inhibitor-based assay: Mueller Hinton agar (MHA) plates were inoculated with the bacterial isolate. Cefoxitin-BA disks were prepared as per Coudron.[10] Cefoxitin and cefoxitin with BA disks were placed on the inoculated MHA plates and incubated overnight at 37°C. An isolate that demonstrated a zone diameter of 5 mm or more in the presence of cefoxitin with BA in comparison with cefoxitin alone was considered an AmpC producer (►Fig. 1).

- Representation of inhibitor-based assay. A pure AmpC-producing isolate showing cefoxitin (FOX) zone enhancement (≥5 mm) with the addition of BA. BA, boronic acid.
Disk approximation test: MHA plates were inoculated with the study isolate. A ceftazidime disk (30 μg) was placed at the center of the inoculated plate. Imipenem (10 μg), cefoxitin (30 μg), and amoxicillin/clavulanate (20/10 μg) disks were then placed at a distance of 20 mm from the ceftazidime disk. The inoculated plates were incubated overnight at 37°C. After overnight incubation, if the isolate demonstrates an obvious blunting or flattening of the zone of inhibition between the ceftazidime disk and the inducing substrates (imipenem, cefoxitin, and amoxicillin/clavulanate disk) then the isolate was considered as an AmpC producer[11] (►Fig. 2).

- Representation of disk approximation test. Flattening of the zone of ceftazidime toward imipenem disk and cefoxitin disk showing AmpC producer. IMP, imipenem (10 μg); FOX, cefoxitin (30 μg); CAZ, ceftazidime (10 μg); AMC, amoxicillin-clavulanate (20/10 μg).
Molecular characterization of AmpC β-lactamase: Multiplex polymerase chain reaction (PCR) was used to detect the most common plasmid-mediated AmpC genes: ACC, FOX, MOX, DHA, CIT, and EBC.[11]
The protocol for multiplex was as follows: for the detection of MOX gene, 5GCTGCTCAAGGAGCACAGGAT-3 was used as forward primer and 5-CACATTGACATAGGTGTGGTGC-3 was used as the reverse primer, expected amplicon size 520 bp. For the detection of CIT gene, 5-TGGCCAGAACTGACAGGCAAA-3 was used as forward primer and 5-TTTCTCCTGAACGTGGCTGGC-3 was used as the reverse primer, expected amplicon size 462 bp. For the detection of DHA gene, 5-AACTTTCACAGGTGTGCTGGGT-3 was used as forward primer and 5-CCGTACGCATACTGGCTTTGC-3 was used as the reverse primer, expected amplicon size 405 bp. For the detection of ACC gene, 5-AACAGCCTCAGCAGCCGGTTA-3 was used as forward primer and 5-TTCGCCGCAATCATCCCTAGC-3 was used as the reverse primer, expected amplicon size 346 bp. For the detection of EBC gene, 5-TCGGTAAAGCCGATGTTGCGG-3 was used as forward primer and 5-CTTCCACTGCGGCTGCCAGTT-3 was used as the reverse primer, expected amplicon size 302 bp. For the detection of FOX gene, 5-AACATGGGGTATCAGGGAGATG-3 was used as forward primer and 5-CAAAGCGCGTAACCGGATTGG-3 was used as the reverse primer, expected amplicon size 190 bp.
DNA extraction was by Modified Proteinase K method.[12] For PCR assays, 2-μL cDNA was added to 23-μL master mixture of PCR reagents. The reaction was programmed with initial denaturation step at 94°C for 3 minutes; followed by 25 cycles of DNA denaturation at 94°C for 30 seconds, primer annealing at 64°C for 30 seconds, primer extension at 72°C for 1 minute; and a final extension step at 72°C for 7 minutes[6] (Department of Molecular Biology and Immunology, MMNGH Institute of Dental Sciences and Research Centre, Belgaum, for Multiplex PCR). Amplified products were subjected to electrophoresis through 3% Agarose gel. 16 µL of each amplified product was loaded into each well. The gel was visualized under UV light illuminator and analyzed using Gel Documentation System (Major Science, California, United States). Negative control used was PCR mix with distilled water and a 100 base-pair DNA ladder was used as the size reference (►Fig. 3).
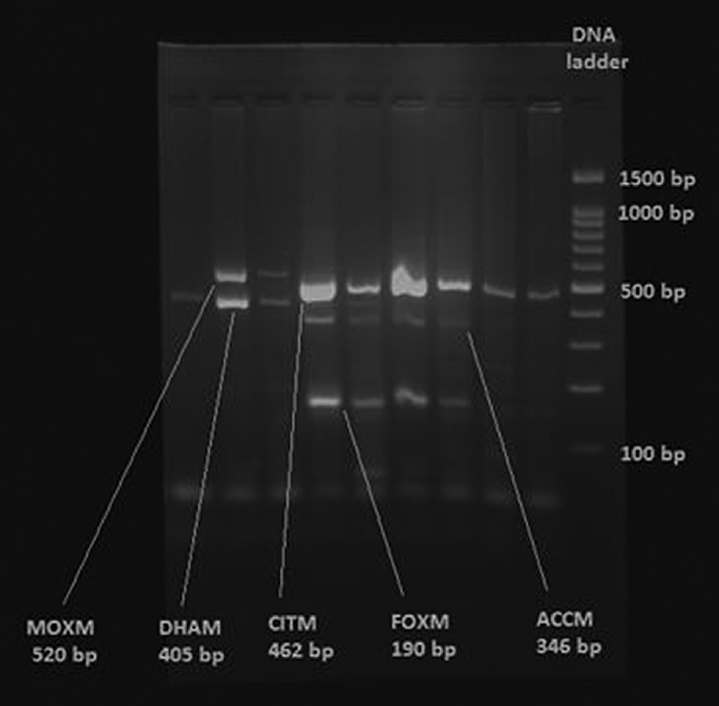
- AmpC genes by multiplex PCR. PCR, polymerase chain reaction.
Statistical analysis: Statistical Package for the Social Science version 20 (IBM, Armonk, New York, United States) was employed to obtain descriptive data.
Results
Antimicrobial susceptibility testing: The clinical isolates showed resistance to multiple antimicrobial drugs. All our isolates showed complete resistance to ampicillin (100%). This was found to coexist with resistance to two or more antimicrobials that is cotrimoxazole (47%), norfloxacin (30.6%), ciprofloxacin (23.1%), and gentamicin (15.5%). Least resistance was observed with amikacin (1.1%) and piperacillin/tazobactam (2.2%).
Phenotypic detection of AmpC β-lactamases: Among 58 (42.3%) cefoxitin-resistant isolates, 53.4 and 18.9% of the isolates were positive by inhibitor-based assay and disk approximation test (►Table 1).
| Isolates | Cefoxitin screening | Inhibitor-based assay | Disk approximation test |
|---|---|---|---|
| Escherichia coli (n = 60) | 28 (46.6%) | 14 (23.3%) | 5 (8.3%) |
| Klebsiella pneumoniae (n = 60) | 23 (38.3%) | 13 (21.6%) | 5 (8.3%) |
| Proteus species(n = 10) | 4 (40%) | 3 (30%) | 1 (10%) |
| Citrobacter koseri (n = 5) | 2 (40%) | 1 (20%) | 0 (0%) |
| Salmonella typhi (n = 1) | 0 (0%) | 0 (0%) | 0 (0%) |
| Enterobacter species (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) |
| Total (n = 137) | 58 (42.3%) | 31 (22.6%) | 11 (8%) |
PCR detection of AmpC genes: Overall, of 137 Enterobacteriaceae members, 42 (30.6%) isolates were positive for AmpC gene subtypes (►Table 2).
| Isolates | AmpC genotype positive |
|---|---|
| Escherichia coli (n = 60) | 17 |
| Klebsiella pneumoniae (n = 60) | 19 |
| Proteus species (n = 10) | 3 |
| Citrobacter koseri (n = 5) | 2 |
| Enterobacter species (n = 1) | 1 |
| Total n = 137 | 42 |
Comparison of phenotypic test results with PCR detection of AmpC genes: Of the 42 isolates with Amp genes detected by multiplex PCR, inhibitor base assay detected 25 (59.5%) isolates, while disk approximation test detected 9 (21.4%) isolates. ►Table 3 shows the statistical analysis of inhibitor-based assay and disk approximation test in comparison with gold standard PCR assay.
| Statistic | Inhibitor-based assay | Disk approximation test | ||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| Sensitivity | 58.14% | 42.13–72.99% | 21.43% | 10.30–36.81% |
| Specificity | 60.00% | 32.29–83.66% | 87.50% | 61.65–98.45% |
| Positive likelihood ratio | 1.45 | 0.74–2.84 | 1.71 | 0.41–7.09 |
| Negative likelihood ratio | 0.70 | 0.41–1.20 | 0.90 | 0.70–1.15 |
| Positive predictive value | 80.65% | 68.08–89.06% | 81.82% | 52.10–94.90% |
| Negative predictive value | 33.33% | 22.51–46.25% | 29.79% | 24.96–35.11% |
| Accuracy | 58.62% | 44.93–71.40% | 39.66% | 27.05–53.36% |
Abbreviation: CI, confidence interval.
Discussion
Plasmid-mediated AmpC resistance pose a big challenge to infection control as the AmpC genes are expressed in higher amounts and are highly transmissible to other bacterial species.[13] Worldwide prevalence of AmpC resistance is unknown, due to the limited number of surveillance studies and lack of laboratory diagnostic techniques in accurately detecting this resistance mechanism.[14] Detection of AmpC β-lactamases is clinically important so as to avoid therapeutic failures and nosocomial outbreaks.[4] Therapeutic options for AmpC producers are limited due to resistance to most of the β-lactam drugs except for cefepime and carbapenem.[15]
In the present study, the clinical isolates showed complete resistance to ampicillin (100%) followed by cotrimoxazole (47%), norfloxacin (30.6%), ciprofloxacin (23.1%), and gentamicin (15.5%). Least resistance was observed with amikacin (1.1%) and piperacillin/tazobactum (2.2%). Similar antimicrobial resistance patterns have been observed among Indian Enterobacteriaceae clinical isolates,[16,17] whereas a study from Romania has reported high antimicrobial resistance against cotrimoxazole (74%), fluoroquinolones (49%), and penicillin (44%). Another study from Iraq has reported high MDR rate with β-lactams, aminoglycosides, and fluoroquinolones among Enterobacteriaceae.[18] Studies in different parts of the world have shown different patterns of antimicrobial resistance. This may be due to inappropriate ignorance and overuse of antibiotics, inappropriate infection control, and lack of awareness of the clinical outcome of multidrug-resistant bacterial infections.[16]
The present study demonstrated that among 58 cefoxitin-resistant isolates, 42 (72.4%) were found to possess AmpC genes by PCR. Similar results were reported by Yilmaz et al[19] and Helmy and Wasfi.[20] Not all cefoxitin-resistant isolates were AmpC producers. Cefoxitin resistance can be due to the presence of other antimicrobial-resistant mechanisms like extended spectrum beta lactamases, metallo beta lactamases, and mutation of porin channels. Cefoxitin also acts as a substrate to active efflux pumps in clinical strains.[20]
Phenotypic methods are unable to differentiate chromosomal and plasmid AmpC β-lactamases. Plasmid AmpC genes are detected by PCR analysis. But the molecular test is expensive and not available for routine use in all the clinical laboratories.[1] Hence there is a practical need for a simple and cost effective assay to detect plasmid AmpC β-lactamases. Our study compared two phenotypic methods and observed 53.4 and 18.9% of the cefoxitin-resistant isolates positive by inhibitor-based assay and disk approximation test. Plasmid AmpC genes were detected in 25 (59.5%) of the 31 inhibitor-based assay positive isolates and 9 (21.4%) of the 11 disk approximation test positive isolates by molecular analysis. Based on these findings, the inhibitor-based assay exhibited 58% sensitivity and 60% specificity and disk approximation test exhibited 21% sensitivity and 87% specificity.
A study from the United States has noted 58% of the boronic acid disk test positive isolates gave positive result using multiplex PCR.[21] In contrast, Yilmaz et al reported the presence of AmpC genes in 22% of the boronic acid positive isolates and the inhibitor-based assay revealed 100% sensitivity and 66% specificity.[19] A study from Egypt reported 100% positivity among 50/148 (33.8%) AmpC isolates, by disk approximation test which was inconsistent with our observation.[11]
False positive phenotypic test results encountered in our study may be due to the presence of unknown AmpC genes or the inability of the phenotypic methods to differentiate chromosomal and plasmid AmpC genes. On the other hand, the reason for the false negative results is the ineffective phenotypic AmpC gene expression.[20] The difference between the phenotype methods and the molecular test results can be explained by the presence of chromosomal AmpCs or porin mutations.[19]
Conclusion
Our findings suggest that inhibitor-based assay using BA is a practical and efficient method for the identification of AmpC producers. This method is cost effective, simple to perform, and easy to interpret. Therefore, inhibitor-based assay using BA can be used for the detection of the isolates that harbor AmpC enzymes in the clinical laboratory where multiplex PCR is not affordable and thus help in therapeutic intervention, improved infection control and prevent the dissemination of antimicrobial resistance.
Availability of data and materials: All data generated or analyzed during this study are included in this article.
Ethical Approval
This research is approved by Annamalai Institutional Review Board.
Authors’ Contribution
R.M.P. contributed towards concept, design, literature search, experimental studies, data acquisition, data analysis, statistical analysis, and manuscript preparation. G.S. did the data analysis, manuscript editing, and manuscript review. K.M.B. contributed towards data analysis, manuscript editing, and manuscript review.
Financial Disclosure
None.
Conflict of Interest
None declared.
References
- Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(03):969-976.
- [CrossRef] [PubMed] [Google Scholar]
- Group ARL; Antibacterial Resistance Leadership Group. A Primer on AmpC β-lactamases: necessary knowledge for an increasingly multidrug-resistant world. Clin Infect Dis. 2019;69(08):1446-1455.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of phenotypic tests for the detection of AmpC beta-lactamase in clinical isolates of Escherichia coli. Indian J Pathol Microbiol. 2013;56(02):135-138.
- [CrossRef] [PubMed] [Google Scholar]
- Genotypic and phenotypic detection of AmpC β-lactamases in Enterobacter spp. isolated from a teaching hospital in Malaysia. PLoS One. 2016;11(03):0150643.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of AmpC lactamases in gram-negative bacteria. J Lab Physicians. 2014;6(01):1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid detection of extended-spectrum β-lactamases (ESBL) and AmpC β-lactamases in Enterobacterales: development of a screening panel using the MALDI-TOF MS-based direct-on-target microdroplet growth assay. Front Microbiol. 2019;10(13):13.
- [CrossRef] [PubMed] [Google Scholar]
- Multidrug resistant Enterobacteriaceae and extended spectrum β-lactamase producing Escherichia coli: a cross-sectional study in National Kidney Center, Nepal. Antimicrob Resist Infect Control. 2015;4(01):42.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic detection and antibiogram of β-lactamase-producing Proteus species in a Tertiary Care Hospital, India. Ann Med Health Sci Res. 2016;6(05):267-273.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitor-based methods for detection of plasmid-mediated AmpC beta-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J Clin Microbiol. 2005;43(08):4163-4167.
- [CrossRef] [PubMed] [Google Scholar]
- Occurrence and detection of AmpC β-lactamases among Enterobacteriaceae isolates from patients at Ain Shams University Hospital. Egypt J Med Hum Genet. 2015;16(03):239-244.
- [CrossRef] [Google Scholar]
- Characterization of FOX-3, an AmpC-type plasmid-mediated beta-lactamase from an Italian isolate of Klebsiella oxytoca. Antimicrob Agents Chemother. 1998;42(02):464-467.
- [CrossRef] [PubMed] [Google Scholar]
- Controversies about extended-spectrum and AmpC beta-lactamases. Emerg Infect Dis. 2001;7(02):333-336.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40(06):2153-2162.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic susceptibility pattern of Enterobacteriaceae and non-fermenter gram-negative clinical isolates of microbial resource orchid. J Nat Sci Biol Med. 2015;6(01):198-201.
- [CrossRef] [PubMed] [Google Scholar]
- Multidrug-resistant Enterobacteriaceae including metallo-β-lactamase producers are predominant pathogens of healthcare-associated infections in an Indian teaching hospital. Indian J Med Microbiol. 2011;29(01):22-27.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotics resistance patterns among Enterobacteriaceae isolated from different clinical samples. Drug Invention Today. 2019;12(05):938-942.
- [Google Scholar]
- Detection of plasmid-mediated AmpC β-lactamase in Escherichia coli and Klebsiella pneumoniae. Indian J Med Microbiol. 2013;31(01):53-59.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic and molecular characterization of plasmid mediated AmpC β-lactamases among Escherichia coli, Klebsiella spp., and Proteus mirabilis isolated from urinary tract infections in Egyptian hospitals. BioMed Res Int. 2014;2014:171548.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of plasmid-mediated AmpC beta-lactamases in Escherichia coli, Klebsiella spp., and proteus species can potentially improve reporting of cephalosporin susceptibility testing results. J Clin Microbiol. 2009;47(02):294-299.
- [CrossRef] [PubMed] [Google Scholar]






