Translate this page into:
Expression of bromodomain-containing 4 in nasopharyngeal carcinoma: Clinicopathology, prognosis, immunity, and regulatory networks
*Corresponding authors: Xianlu Zhuo, Department of Otolaryngology, Head and Neck Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou, China. zhuoxianlu@gmc.edu.cn
Houyu Zhao, Department of Otolaryngology, Head and Neck Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou, China. zhaohouyu@gmc.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Sun Z, Guo X, Feng B, Chen H, Ling J, Chang A, et al. Expression of bromodomain-containing 4 in nasopharyngeal carcinoma: Clinicopathology, prognosis, immunity, and regulatory networks. J Lab Physicians. 2024;16:483-95. doi: 10.25259/JLP_39_2024
Abstract
Objectives:
Nasopharyngeal carcinoma (NPC) is a highly aggressive, insidious, recurrent, and metastatic head-and-neck malignancy with a poor prognosis. Bromodomain-containing 4 (BRD4) was upregulated in various tumors and was associated with poor prognosis. Therefore, this study aimed to investigate the biological function of BRD4 in NPC and the molecular mechanisms of expression regulation.
Materials and Methods:
Based on biological big data, the expression, clinical significance, and possible biological functions of BRD4 in head-and-neck cancer were explored by bioinformatics. Then, based on a tissue microarray containing a cohort of NPC, BRD4 protein expression was detected by immunohistochemistry to explore its clinical significance and impact on prognosis.
Statistical analysis:
A p-value less than 0.05 was considered as significant.
Results:
BRD4 expression was upregulated in NPC and positively correlated with metastasis, higher tumor grades, and clinical stages, which might be positively correlated with copy number variation, CD4+ T cells, and immune checkpoint suppressor genes. Moreover, the sensitivity of cancer cells to paclitaxel and gemcitabine was negatively correlated with BRD4 expression. In addition, immunohistochemical staining showed that BRD4 was overexpressed in NPC tissues, which was correlated with lymph node metastasis and poor clinical outcomes.
Conclusions:
BRD4 is highly expressed in NPC tissues and is associated with a poor prognosis. Its aberrant expression may be closely linked to alterations in the immune microenvironment and chemotherapeutic resistance. Moreover, BRD4 is not only an oncogene in NPC but also a potential therapeutic target.
Keywords
Nasopharyngeal carcinoma
Bromodomain-containing 4
Immunohistochemistry
Prognosis
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a highly malignant head-and-neck cancer (HNC) originating from the epithelial tissue of the nasopharynx.[1] Epidemiological surveys have shown that the incidence of NPC was higher in Southeast Asia, southern provinces of China, and North Africa and was strongly associated with Epstein–Barr virus (EBV) infection, environmental factors, and genetic susceptibility.[2,3] Approximately 130,000 new cases of NPC and 80,000 deaths were reported worldwide in 2020.[4] More than 70% of patients with NPC were already at a locally advanced stage at the time of initial diagnosis because of the asymptomatic nature of NPC in the early stage.[5] Moreover, owing to missing the optimal early radiotherapy timing, the prognosis of NPC patients was very unsatisfactory.[6] Therefore, there was an urgent need to explore new biomarkers of NPC along with understanding the underlying molecular mechanisms, which were crucial for developing effective diagnostic and therapeutic strategies.
Bromodomain-containing 4 (BRD4), which belongs to the bromodomain and extraterminal protein family, was an essential transcriptional and epigenetic regulator that performed pivotal roles in the development of many cancers.[7] For instance, the expression of BRD4 was increased in patients with esophageal squamous cell carcinoma, and the results of mouse experiments showed that targeting BRD4 with inhibitors reduced tumor volume.[8] Similarly, BRD4 was found to be overexpressed in patients with colorectal cancer, and a strategy involving the knockdown of BRD4 was effective in inhibiting tumor cell proliferation and inducing apoptosis.[9] Moreover, in bladder cancer, BRD4 was detected to be upregulated in tissues and cells, and BRD4 also promoted cell migration and invasion by positively regulating the Sonic Hedgehog signaling pathway. [10] Based on the above evidence, overexpression of BRD4 might have facilitated the malignant transformation of cancer through different mechanisms of action, such as proliferation, metastasis, invasion, and Epithelial-mesenchymal transition (EMT), and resulted in poor prognosis.
Although the above studies have suggested a strong association of BRD4 in cells with the malignant progression of multiple diseases, the specific mechanism of its action in NPC remained unclear, prompting us to investigate its potential diagnostic and therapeutic value in NPC. Therefore, we performed a comprehensive analysis of immunohistochemistry and some data from public databases to elucidate further the potential mechanism of BRD4’s role in NPC and explored its essential role in its progression.
MATERIALS AND METHODS
Expression level of BRD4
Initially, the present study entailed exploring the expression of BRD4 in pancreatic cancer through the utilization of the TIMER 2.0 online tool http://timer.cistrome.org/. Subsequently, the expression levels of BRD4 in HNC tissues in comparison to normal tissues were assessed based on information gathered from the HNC cohort data, which were obtained from the cancer genome atlas (TCGA) database (https://portal.gdc.cancer.gov/) and evaluated using the University of Alabama at Birmingham Cancer data analysis Portal (UALCAN) database (https://ualcan.path.uab.edu/). Next, the NPC public gene expression profile (GSE61218) was obtained from the gene expression omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) and analyzed using R software to evaluate the expression levels of BRD4 in NPC tumors and normal tissues. Ultimately, the expression of the BRD4 gene and its relationship with HNC metastasis were evaluated using the TNMplot tool (https://tnmplot.com/analysis/).
Correlation of BRD4 expression levels with clinicopathological features
The present study employed the UALCAN database to examine the potential associations between BRD4 gene expression in tumor tissue and several clinical parameters, encompassing patient age, gender, disease stage, and lymph node metastasis (LNM).
Genetic variation analysis of BRD4
The mutation rate, mutation frequency, and mutation loci of BRD4 in HNC (TCGA, Nature 2015) were explored using the cBioPortal database (https://www.cbioportal.org/).
The mutation of the BRD4 gene in cancer was investigated by utilizing the catalog of somatic mutations in Cancer (COSMIC) database (https://cancer.sanger.ac.uk/cosmic).
The present study investigates the potential influence of copy number variation (CNV) in the BRD4 gene on the clinical prognosis of HNC patients, utilizing the gene set cancer analysis (GSCA) database (https://guolab.wchscu.cn/GSCA/#/).
Correlation analysis of BRD4 expression and immunity
To investigate the relationship between BRD4 gene expression and tumor immune cell infiltration, the HNC cohort data based on the TCGA database were downloaded from the UCSC platform (https://xenabrowser.net/). The gene expression profiles of BRD4 were further extracted from the samples of HNC. Three different algorithms, TIMER, CIBERSORT, and Estimating the proportion of immune and cancer cells (EPIC), in R software were further utilized to assess the correlation between BRD4 gene expression and immune cell infiltration in each patient with HNC.
In addition, to assess the correlation between BRD4 and immune modulators in HNC, data from HNC and normal samples based on the TCGA database were downloaded from the UCSC platform. Moreover, the expression data of the BRD4 gene and immune checkpoint pathway genes (Inhibitory, sourced from the literature “The Immune Landscape of Cancer”[11]) were extracted from various samples of HNC. Samples from primary solid tumor, primary tumor, primary blood-derived cancer-bone marrow, and primary blood-derived cancer-peripheral blood were further screened. Furthermore, all normal samples were filtered, and log2(x+0.001) transformations were applied to each expression value. Next, the Pearson correlation between BRD4 and marker genes of the five immune pathways was calculated.
Intergenic interaction prediction for BRD4
To elucidate the biological function of the BRD4 gene, the Networkanalyst (https://www.networkanalyst.ca/) database was utilized to predict the target genes of BRD4. After that, gene ontology (GO) analysis (https://www.geneontology.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://www.genome.jp/kegg/) pathway enrichment analysis were executed using the DAVID database (https://david.ncifcrf.gov/) to assess these target genes’ potential functions and gain further insight into the possible roles of BRD4.
Drug sensitivity prediction
The cancer therapeutic response portal (CTRP) (https://portals.broadinstitute.org/ctrp/) was used to predict the possible impact of BRD4 expression on drug sensitivity, and GSCA was used to help visualize the results.
Tissue microarray
A tissue microarray (HNasN129Su01) containing 129 NPC samples was provided by Shanghai Outdo Biotech Co., Ltd. The study was approved by the Ethics Committee of Shanghai Outdo Biotech Co. Ltd. (SHYJS-CP-1810011). This group of patients with NPC consisted of 99 males and 30 females, aged between 20 and 82 years (median age: 47 years). Patients were diagnosed between January 2010 and October 2011, with the final follow-up performed in March 2017. All cases underwent surgery, had no prior chemotherapy or radiotherapy, and were pathologically and clinically diagnosed with NPC.
Immunohistochemistry
Immunohistochemistry was used to detect the expression of BRD4 protein. The tissue sections, which were fixed in formalin and embedded in paraffin, were heated in an oven at 63°C for 1 h. Xylene was used as a solvent for paraffin. An automated immunohistochemistry staining instrument (Leica Biosystems, LEICAST5020, USA) was used for dewaxing and hydration. Antigen recovery was performed using an automated immunohistochemistry processing instrument (Dako, PT Link, Denmark). After recovery, the sections were placed in distilled water at room temperature and allowed to cool naturally for more than 10 min. BRD4 expression was detected with an anti-BRD4 antibody (Dako, S3022, Denmark) at a dilution of 1:300 with the use of an automated immunohistochemistry staining instrument (Dako, Autostainer Link 48, Denmark). The sections were counterstained with hematoxylin, dehydrated, and sealed with neutral resin.
Interpretation of immunohistochemistry staining
Immunohistochemistry staining was interpreted using the comprehensive scoring method[12] which combines the intensity and percentage of positive cells for assessment. Staining intensity was classified on a scale from 0 to 3, with 0 indicating negativity, 1 indicating weakness, 2 indicating moderation, and 3 indicating strength. The staining percentage was rated on a scale from 0 to 4, with 1 representing 0–25%, 2 representing 26–50%, 3 representing 51–75%, and 4 representing 76–100%.
The results were independently evaluated by two pathologists who were unaware of the individual’s clinical data as well as histological and histopathological information. When scoring the intensity and positive percentage of the samples, the total score was obtained by multiplying these two parameters. Therefore, the total score ranged from 0 to 12. Based on pre-defined score thresholds, a score ≥6 indicated high expression, while a score <6 indicated low expression.
Statistical analysis
For continuous variables, t-tests, analysis of variance, or Wilcoxon rank-sum tests were used to assess between-group differences, depending on the specific data type. For ratio comparisons, the Chi-square test was chosen. Overall, survival curves were calculated using the Kaplan–Meier method, and differences in survival rates were evaluated using the log-rank test. Cox multivariate regression analysis was performed to consider all possible clinical factors. Analyses were conducted using MedCalc software (15.2.2; Mariakerke, Belgium). P < 0.05 was considered statistically significant.
RESULTS
The abnormal expression of BRD4 in HNC/NPC
Abnormal gene expression in tumor tissues may be involved in tumorigenesis and progression. As shown in Figure 1a, the BRD4 was significantly upregulated in various tumor tissues such as BRCA, CHOL, COAD, ESCA, and HNSC. TCGA HNSC data showed that there was significant overexpression of BRD4 in HNC samples compared with adjacent normal tissues (P < 0.05), [Figure 1b]. Next, based on the GEO database GSE61218, BRD4 was also detected as significantly upregulated in NPC tumor tissues compared to normal tissues (P < 0.05), [Figure 1c].
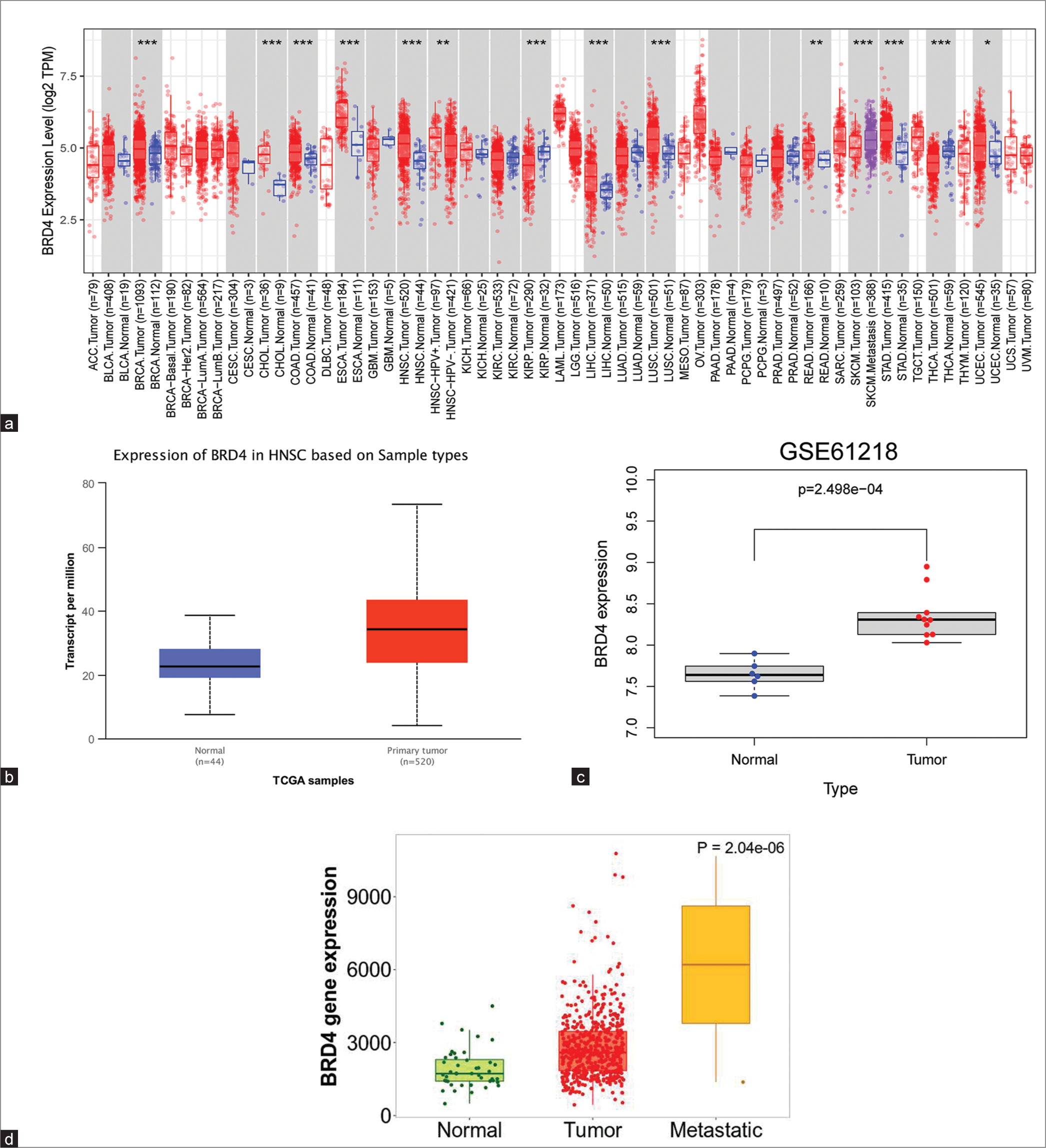
- Expression of bromodomain-containing 4 (BRD4) in head-and-neck cancer (HNC)/nasopharyngeal carcinoma (NPC). (a) BRD4 gene expression in pancreatic cancer was assessed using the cancer genome atlas database cohorts. (b) BRD4 expression was significantly higher in HNC tissues than in normal tissues (P < 0.05). (c) BRD4 expression was upregulated in NPC (P < 0.05). (d) The expression of BRD4 in metastatic tissues of HNC was significantly higher compared to primary tumor tissues and normal tissues (P < 0.05). *P < 0.05; **P < 0.01; ***P < 0.001. TPM: Transcripts per million, HNSC: Head and neck squamous carcinoma, TCGA: The cancer genome atlas.
In addition, the results of the TNMplot tool indicated that increased expression of BRD4 may have been associated with enhanced metastatic ability of HNC cells (P < 0.05), [Figure 1d].
Correlation of BRD4 expression levels with different clinical characteristics
The UALCAN database explored the correlation of BRD4 expression levels with different clinical characteristics in HNC. The results showed that BRD4 expression had no statistical significance with age (P > 0.05), [Figure 2a]. BRD4 expression was found to be higher in male patients compared to female patients (P < 0.05), [Figure 2b]. BRD4 expression levels were higher in stage IV patients compared with stage III patients, and the difference was statistically significant (P < 0.05), [Figure 2c]. Similarly, BRD4 expression levels were elevated in patients with G2 and G3 compared with G1 (P < 0.01), [Figure 2d]. The above results suggest that higher BRD4 expression in HNC was significantly associated with males, higher tumor grade, and stage. However, there was no statistical significance between the expression level of BRD4 and N stage [Figure 2e], as well as TP53 mutation [Figure 2f] (P > 0.05).
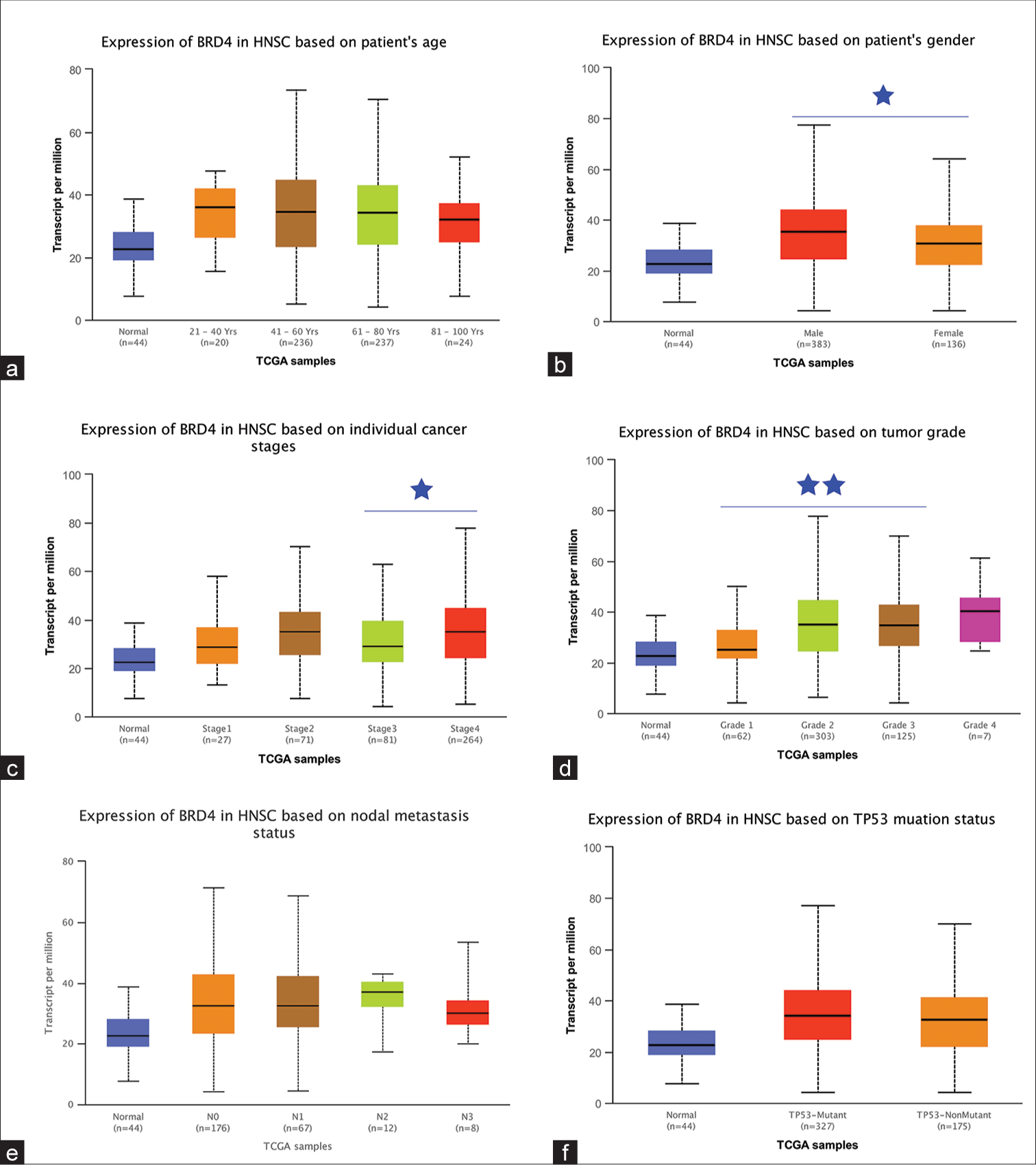
- Relationship between bromodomain-containing 4 (BRD4) expression in head-and-neck cancer (HNC) and clinicopathological features. Box plots represented the expression of BRD4 in different HNC subtype samples by (a) age, (b) gender, (c) stage, (d) grade, lymph node metastasis, and (f) TP53 mutation status, respectively. NS: Not statistically significant. *P < 0.05; **P < 0.01. TCGA : The cancer genome atlas, TP53 : Tumor protein P53
Analysis of BRD4 gene mutations and CNV
To explore the possible underlying mechanisms of BRD4 gene expression abnormalities in HNC, mutation and CNV analyses were performed. The results showed that the frequency of BRD4 gene alteration was 3% in 279 HNC patients [Figure 3A(a)], which mainly included “mutation” and “amplification.” Among the major types of genetic alterations, “mutation” was the most common type of BRD4 gene mutation [Figure 3A(b)]. In addition, to better understand the mutational profile of BRD4 in HNC across the structural domain of the protein, a total of five mutation sites were detected, located between 0 and 1362 [Figure 3A(c)]. Then, further using the catalogue of somatic mutations in cancer (COSMIC) online tool, the primary mutation type of BRD4 was observed as missense substitution (59.40%), with results similar to those of cBioProtal [Figure 3B(a)]. Among them, the main substitution mutation types were C>T (37.35%) and G>A (21.50%) [Figure 3B(b)].
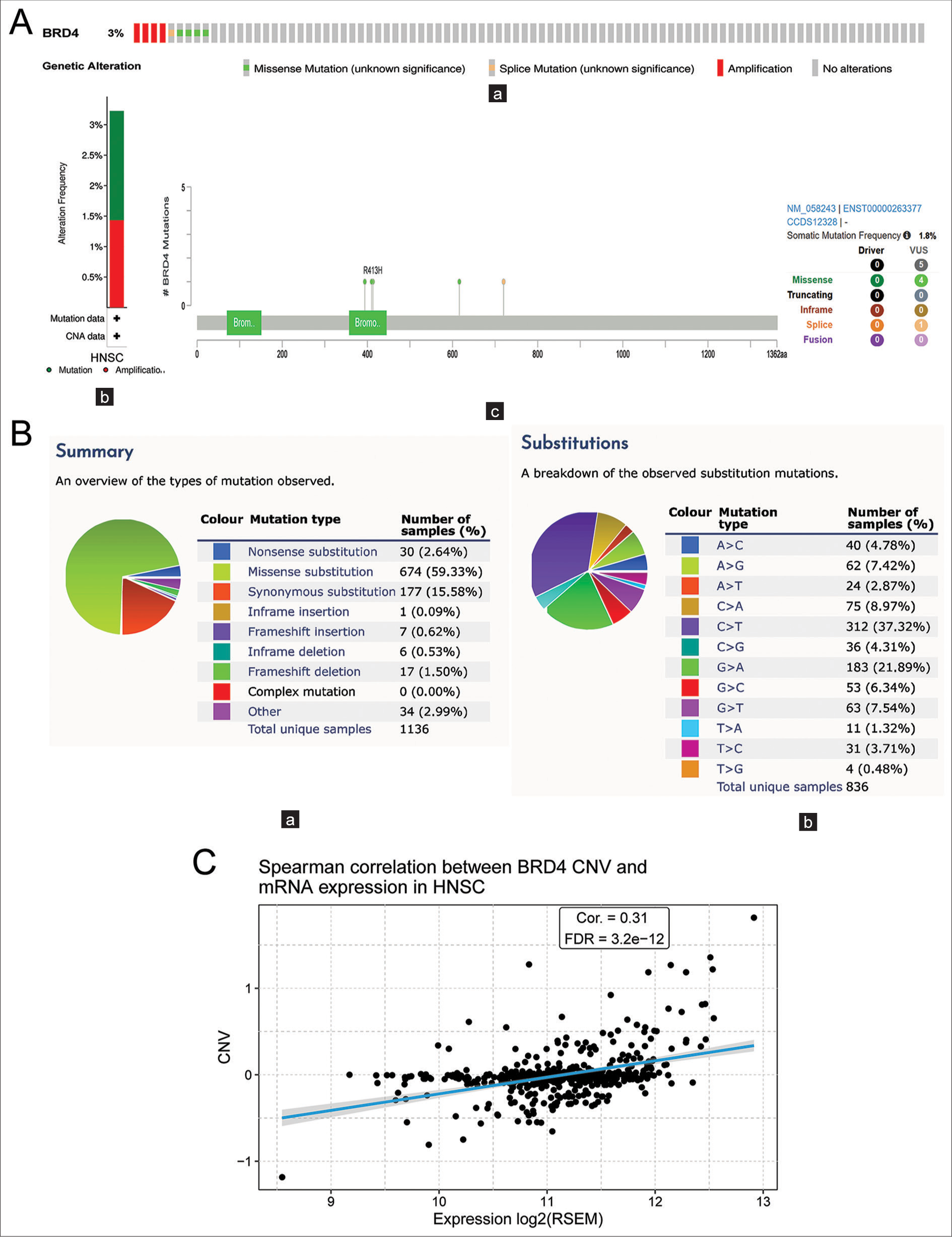
- (A) Analysis of bromodomain-containing 4 (BRD4) genetic alterations and copy number variation (CNV) in head-and-neck cancer (HNC). (a) Total mutation of BRD4 in the HNC genome. (b) Type of BRD4 gene alteration frequency in HNC. (c) Mutation map of BRD4 in HNC across the structural domain of the protein. (B) (a and b) Types of BRD4 gene mutations and substitutions in HNC. (C) Correlation of BRD4 CNV and messenger RNA expression in HNC. BRD: Bromodomain-containing, VUS: Variant of uncertain significance, CAN: Copy number alteration, RSEM: RNA-Seq by expectation-maximization, FDR: False discovery rate, Cor: Correlation.
CNV analysis showed a significant positive correlation between CNV and messenger RNA expression of the BRD4 gene in HNC (P < 0.001), [Figure 3C].
These findings indicated that the aberrant expression of BRD4 in tumors might be caused by genetic alterations and CNV, which would contribute to a comprehensive understanding of the mechanisms of NPC development and guide potential therapeutic strategies.
Correlation analysis of BRD4 gene expression with immune cell infiltration
Assessing the correlation between the levels of various immune cell infiltration and BRD4 expression in HNC contributed to our understanding of the possible role of BRD4 on immunity in tumor patients. The results of their analysis by three immune algorithms, TIMER, EPIC, and CIBERSORT, all showed a significant positive correlation between BRD4 expression and infiltration of CD4+ T cells (P < 0.05), [Figure 4a-c]. Therefore, it was speculated that BRD4 may influence the infiltration of immune cells in the cancer microenvironment.
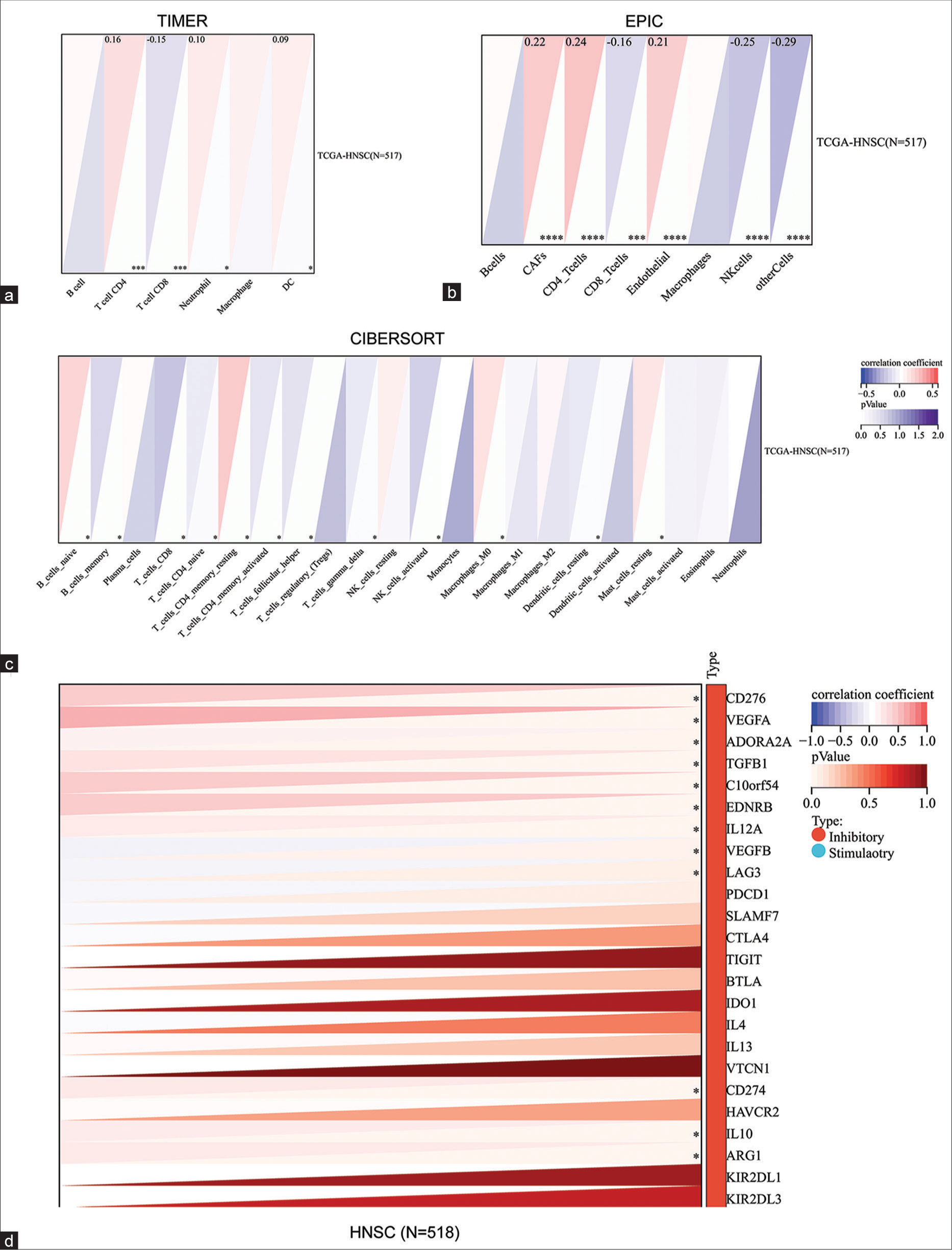
- Correlation analysis of bromodomain-containing 4 (BRD4) and immunity in head-and-neck cancer (HNC). (a) BRD4 expression in HNC was assessed with the infiltration levels of different immune cells using TIMER, (b) EPIC, and (c) CIBERSORT algorithms, respectively. (d) The correlation of BRD4 with 24 immune checkpoint inhibitor-related genes in HNC was assessed. *P < 0.05; ***P < 0.001; ****P < 0.0001. CAF: Cancer-associated fibroblasts, CD: Cluster of differentiation, TGCA: The cancer genome atlas, HNSC: Head and neck squamous carcinoma
Furthermore, more in-depth immunomodulator analysis showed that BRD4 was positively correlated with various suppressive immune checkpoints such as CD274, CD276, and vascular endothelial growth factor (VEGF) in HNC (P < 0.05), [Figure 4d].
Prediction of inter-gene interactions of BRD4
To better understand the possible functions of BRD4, potential targets for its interaction were explored. The results of the analysis showed that a total of 34 genes may interact closely with BRD4 [Figure 5a].
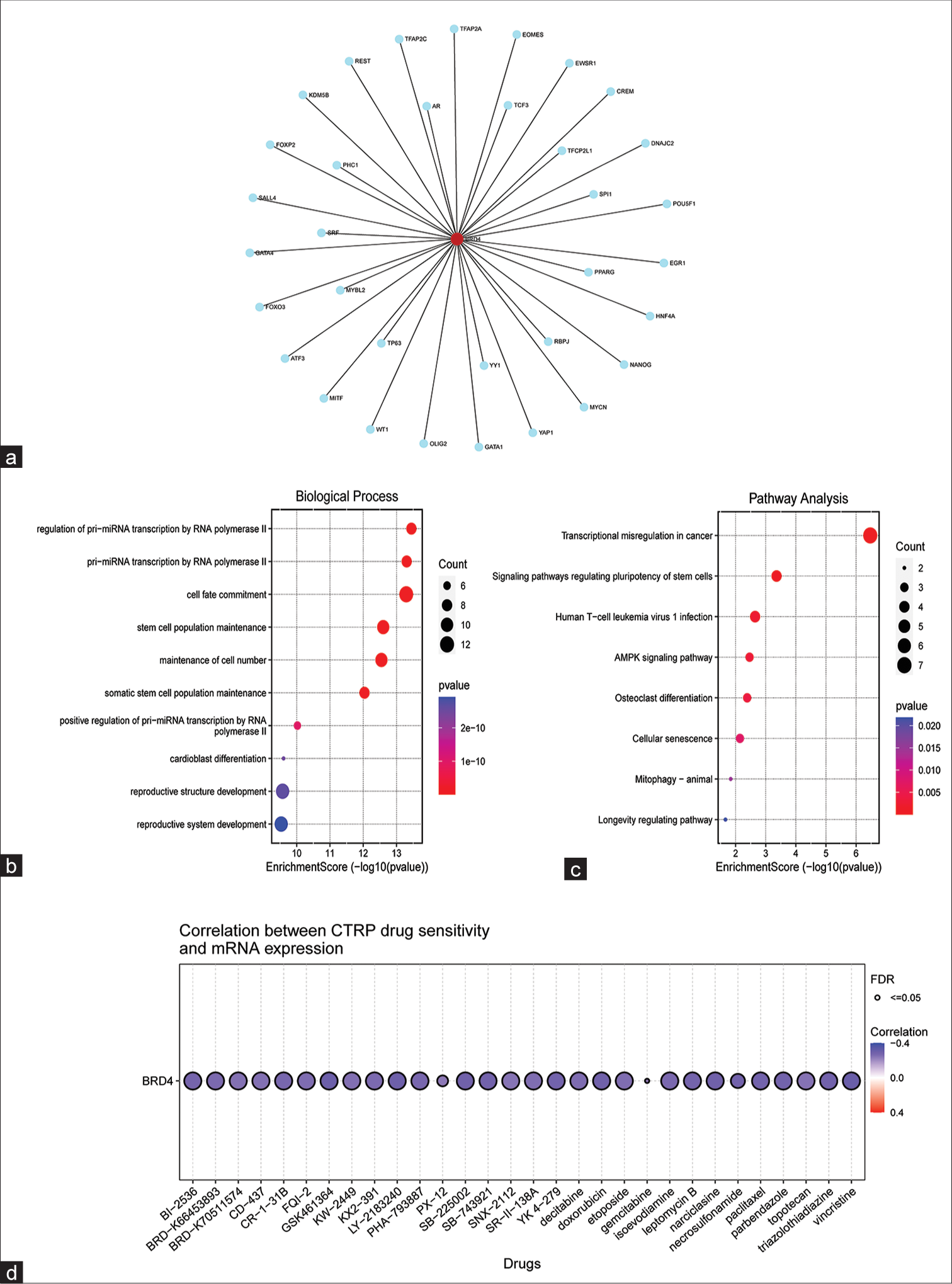
- Predicted target genes of bromodomain-containing 4 (BRD4) and their functional analysis. (a) Networkanalyst online tool predicted that there might be 34 target genes that closely interact with BRD4. (b) Gene ontology enrichment analysis of the 34 target genes. (c) Kyoto encyclopedia of genes and genomes enrichment analysis of 34 target genes. (d) Analysis of BRD4 gene expression on chemotherapeutic drug sensitivity in cancer cells. Blue represents a negative correlation. FDR: False discovery rate. CTRP: The cancer therapeutics response portal, mRNA: Messenger RNA
The GO and KEGG enrichment analysis of these target genes revealed that the top 10 GO terms were mainly focused on the transcriptional regulation of the genes [Figure 5b]. Meanwhile, the top 10 KEGG enrichment results were mainly focused on several aspects: (1) Regulation of signaling pathways: AMPK signaling pathway, stem cell function regulation signaling pathway, etc.; (2) transcriptional regulation of cancer; and (3) effects on cellular processes: Cellular senescence and osteoclast differentiation [Figure 5c].
The data indicated that BRD4 might involve many different signaling pathways and exert oncogenic effects on nasopharyngeal cells through highly complex mechanisms.
Correlation of drug sensitivity of cancer cells with BRD4 expression levels
In this study, an investigation was conducted to examine the potential impact of BRD4 gene expression on the chemosensitivity of cancer cells. The CTRP database was utilized to obtain relevant data. The findings, as depicted in Figure 5d, revealed a negative correlation between the expression of BRD4 and the sensitivity of cancer cells to various drugs, such as decitabine, adriamycin, gemcitabine, paclitaxel, and vincristine. Notably, paclitaxel (r = −0.28, false discovery rate [FDR] <0.001) and gemcitabine (r= −0.24, FDR <0.001) were commonly administered in the clinical treatment of NPC.
BRD4 immunohistochemistry staining results based on NPC tissue microarrays
The BRD4 protein was detected through the use of immunohistochemistry. In tissue microarrays, the specific light brown staining was mainly located in the cytoplasm of tumor cells (P < 0.05), [Figure 6a]. Furthermore, we evaluated the prognostic value of BRD4 in NPC. The survival curves revealed that individuals with substantial BRD4 expression experienced worse clinical outcomes in contrast to those with low expression (P < 0.05), [Figure 6b].
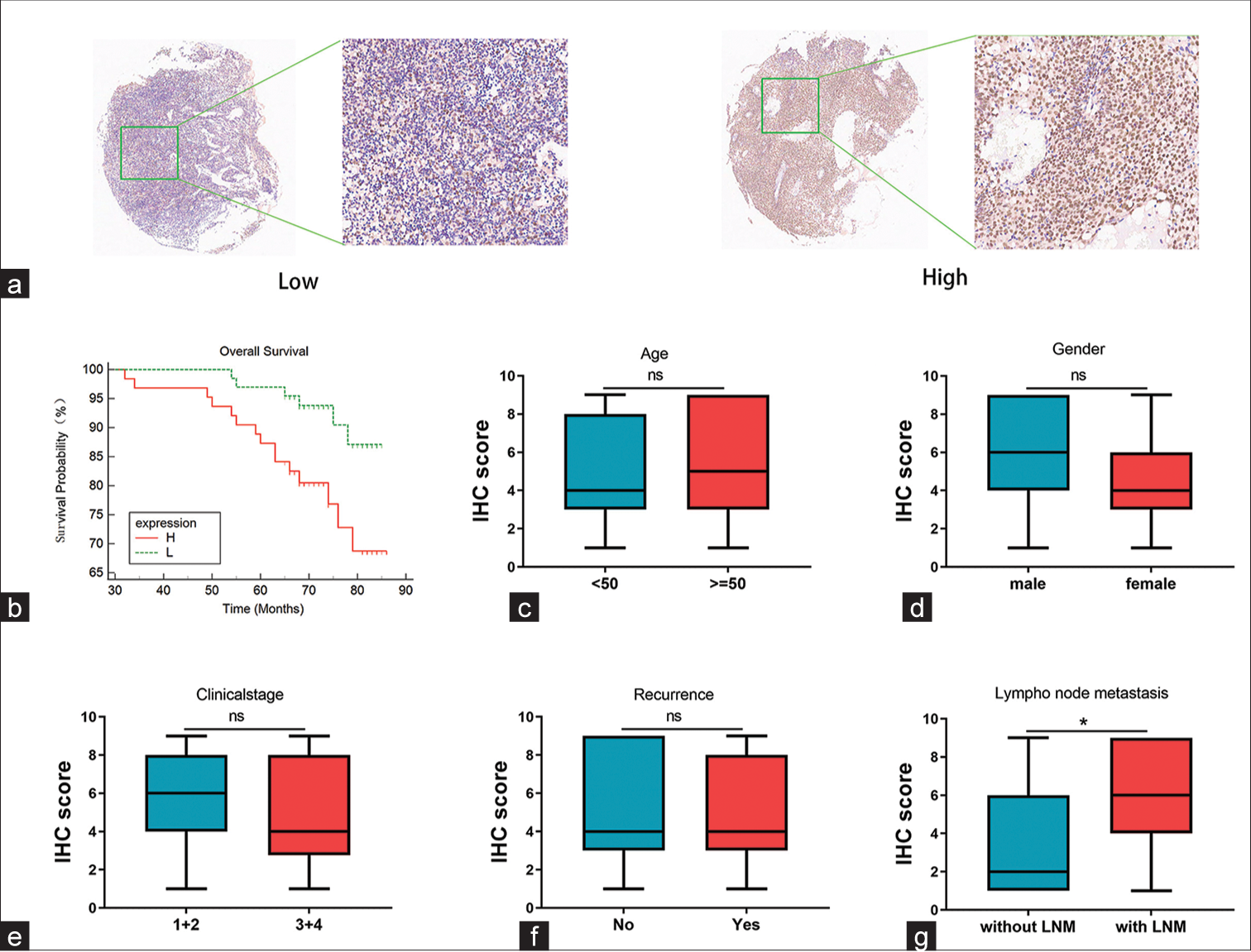
- Nasopharyngeal carcinoma (NPC) microarray-based bromodomain-containing 4 (BRD4) immunohistochemistry staining results. (a) Evaluation of BRD4 protein expression in NPC samples revealed staining predominantly in cancer cell cytoplasm. (b) Survival analysis results demonstrated that patients with high BRD4 expression in NPC had a shorter overall survival time compared to those with low expression (P < 0.05). (c-f) There was no significant correlation between BRD4 expression and age, gender, clinical stage, and recurrence (P > 0.05). (g) Significant correlation was observed between BRD4 and lymph node metastasis (P < 0.05). ns: Not statistically significant, IHC: Immunohistochemistry, LNM: Lymph node metastasis. *P < 0.05.
In addition, the relationship between BRD4 expression and clinicopathological features was evaluated. The study acquired data from five parameters of the NPC cohort: Age, gender, clinical stage, recurrence, and LNM. Moreover, according to the expression of BRD4, it was divided into two expression groups: High and low. The results indicated that high expression of BRD4 was not correlated with age, gender, clinical stage, or recurrence (P > 0.05) [Figures 6c-f], but it may be associated with LNM (P < 0.05) [Figure 6g].
DISCUSSION
NPC, as a malignant tumor of epithelial origin in the nasopharynx, has its unique biological features, epidemiology, and etiology.[13] Although great progress has been made in radiotherapy and chemotherapy in recent years, NPC was often found at an advanced stage, resulting in a poor prognosis.[14] Therefore, the exploration of reliable tumor markers was crucial for the early diagnosis and molecular targeting of NPC. In the present study, we found that overexpression of BRD4 in NPC was significantly associated with enhanced metastasis, higher tumor grades, and stages. Meanwhile, CNV of BRD4, CD4+ T cells, and immune checkpoint suppressor genes also influenced the expression of BRD4. The sensitivity of paclitaxel and gemcitabine was negatively correlated with BRD4 expression. In addition, immunohistochemical staining results demonstrated that high BRD4 expression was detected in NPC tissues.
In recent years, although there were challenges in improving the prognosis of patients with recurrent or metastatic NPC, immunotherapy research for NPC patients has made considerable remarkable progress and exerted its unique antitumor benefits.[15,16] Data from the present study indicated that expression of BRD4 in HNC correlated significantly and positively with CD4+ T cells. The tumor immune microenvironment led to cancer immunosuppression and participated in tumorigenesis and tumor progression.[17] Evidence has demonstrated the importance of CD4+ T cells and their secretory factors for the tumor microenvironment, which may exert both pro- and anti-tumor immune responses.[18] Moreover, some subpopulations of CD4+ T cells may also assist B cells in regulating tissue homeostasis and repairing or performing immune regulation.[19] Previous evidence has indicated that BRD4 may alter the tumor microenvironment to promote tumorigenesis and progression. For example, BRD4 was involved in regulating the microenvironment of lung tumors and had a crucial role in inflammation-mediated tumor metastasis.[20] However, the specific molecular mechanisms of BRD4 in NPC still need to be completely elucidated, and further studies are needed.
Metastasis and recurrence accounted for 95% of deaths in NPC.[21] Particularly, distant metastasis was the main cause of treatment failure and death in NPC.[22] The present study indicated that high expression of BRD4 was also associated with metastasis, as the metastatic tissues of HNC exhibited higher levels of BRD4 compared to the primary cancer tissues and normal tissues. Previous studies had confirmed that BRD4 inhibited Snail polyubiquitination and proteasomal degradation in an acetylation-dependent manner, thereby promoting the progression and metastasis of gastric cancer.[23] In breast cancer, circBCBM1 was involved in brain metastasis through the regulation of the circBCBM1/miR-125a/BRD4 axis.[24] Therefore, based on these studies, we had reason to speculate that enhanced expression of BRD4 promoted the metastasis of NPC cells, and targeting BRD4 might significantly reverse the malignant phenotype of cancer cells.
Cancer immunotherapy with immune checkpoint blockade has revolutionized conventional oncology treatments and achieved favorable clinical responses in treating patients with specific cancer types.[25,26] The present study results showed that in HNC tissues, the expression levels of BRD4 positively correlated with the expression levels of many immune checkpoint inhibitor genes, such as CD276, CD274, VEGFA, and TGFβ1. Much previous evidence suggested that these genes related to immune checkpoint inhibitors may lead to immune escape in NPC through different mechanisms. For example, CD274, also known as programmed death-ligand 1 (PD-L1), is an immunomodulatory protein that serves as an immune checkpoint inhibitor. High expression of PD-L1 in NPC tissues led to a poor prognosis, and PDL1-positive HNC patients receiving immune checkpoint inhibitors had satisfactory treatment outcomes and prognosis.[27,28] As a result, it has been hypothesized that the BRD4 gene might be a potential target for improving the efficacy of immunotherapy in NPC patients, and further experimental validation is needed in the future. In addition, cancer development and progression cannot occur without mutations in the underlying biology of cancer cells, and these mutation patterns may help us understand the mechanisms of disease malignancy at the genetic level.[29] Therefore, we investigated in depth the frequency of mutations in BRD4 in HNC and the association between CNV and BRD4 expression. The findings revealed that the frequency of BRD4 gene alterations amounted to 3%, and the expression of CNV and BRD4 was positively correlated.
The process of BRD4-induced malignant transformation of normal nasopharyngeal epithelial cells was quite complex. GO analysis indicated that the target genes interacting with BRD4 might be mainly related to the transcriptional regulation of genes. KEGG analysis showed that these target genes were mainly enriched in regulating signaling pathways, transcriptional regulation of cancer, and effects on cellular processes. Among them, the signaling pathways include the “AMPK signaling pathway,” “Signaling pathways regulating pluripotency of stem cells,” and so on. As a classical oncogenic signaling pathway, the AMPK signaling pathway facilitated the development of NPC through sophisticated regulatory mechanisms. For example, TIPE1 modulated the AMPK/mTOR signaling pathway by mediating the inhibition of autophagy, which induced the malignant proliferation of nasopharyngeal cells.[30] Similarly, EBV-miR-Bart1-5P targets the α1 catalytic subunit of AMP-activated protein kinase (AMPKα1) and activates the AMPK/mTOR/HIF1 pathway, leading to aberrant aerobic glycolysis and angiogenesis in NPC cells.[31] Moreover, adiponectin inhibited the growth of NPC cells significantly, leading to cell cycle arrest, depending on the AMPK signaling pathway.[32] Therefore, these pieces of evidence offer insights into investigating the role of BRD4 in the development of NPC and require further clarification in future experiments for better understanding. Squamous cell carcinoma was the main pathological type of HNC, and chemotherapy represented an essential tool to improve its patients’ quality of life and prognosis.[33,34] However, the development of drug resistance in tumor cells has been an immeasurable obstacle to the effective treatment of patients, especially NPC.[35] Notably, the results of drug sensitivity analysis through the GSCA website showed that the expression of BRD4 correlated significantly with the sensitivity of paclitaxel and gemcitabine. Moreover, these two chemotherapeutic agents are commonly used in the clinical treatment of NPC and were, therefore, selected for further exploration.[36,37] For example, DDX53 expression was upregulated in paclitaxel-resistant NPC cells, which might be transferred to normal NPC cells through exosomal secretion, thus promoting paclitaxel resistance in NPC cells.[38] Similarly, silencing of hsa_circ_0028007 increased the sensitivity of NPC cell lines (CNE2 and HONE1) to paclitaxel, leading to the speculation that hsa_circ_0028007 may promote their drug resistance.[39] Furthermore, pyruvate dehydrogenase kinase 1 and 2 inhibition reversed gemcitabine resistance in cancer stem cells in HNC.[40] With the above evidence, it has to be said that the mechanisms of paclitaxel and gemcitabine resistance in NPC/HNC patients were extremely complex. Therefore, the resistance mechanism of the BRD4 gene with two chemotherapeutic drugs identified in this study would be attacked in the future, which might favorably prolong the prognosis of patients.
It will be acknowledged that despite our exploration from multiple databases and perspectives, this study had some limitations. First, although the extensive data analysis provided some significant insights into the mechanism of BRD4 causing NPC, more in vitro or in vivo biological experiments for validation remain necessary. Second, considering the geographical nature of NPC development, there was less data available in public databases, leading to limited bioinformatics studies.
CONCLUSIONS
The findings of this study demonstrated well the potential mechanism of action of BRD4 in NPC. This might provide new insights into using BRD4 for the prevention and treatment of NPC.
Author contributions
AC, XZ, HZ: Conception and design;
ZS, JL, HC: Analysis and interpretation of data;
BF, AC, HZ: Statistical analysis;
ZS, XG, XZ: Manuscript editing and revising.
Ethical approval
The research/study was approved by the Ethics Committee of Shanghai Outdo Biotech Co. Ltd. with approval reference number SHYJS-CP-1810011, dated 12th October 2018.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This study was partially supported by the Guizhou Science and Technology Project (ZK2022-044), and the Cultivation project of Affiliated Hospital of Guizhou Medical University (I-2020-10 and gyfybsky-2021-60).
References
- Nasopharyngeal carcinoma: An evolving paradigm. Nat Rev Clin Oncol. 2021;18:679-95.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct Target Ther. 2020;5:245.
- [CrossRef] [PubMed] [Google Scholar]
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of deep learning PET/CT-based radiomics: Potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2019;25:4271-9.
- [CrossRef] [PubMed] [Google Scholar]
- Future of radiotherapy in nasopharyngeal carcinoma. Br J Radiol. 2019;92:20190209.
- [CrossRef] [PubMed] [Google Scholar]
- BRD4 and cancer: Going beyond transcriptional regulation. Mol Cancer. 2018;17:164.
- [CrossRef] [PubMed] [Google Scholar]
- BRD4 drives esophageal squamous cell carcinoma growth by promoting RCC2 expression. Oncogene. 2022;41:347-60.
- [CrossRef] [PubMed] [Google Scholar]
- BRD4 inhibitor AZD5153 suppresses the proliferation of colorectal cancer cells and sensitizes the anticancer effect of PARP inhibitor. Int J Biol Sci. 2019;15:1942-54.
- [CrossRef] [PubMed] [Google Scholar]
- BRD4 promotes the migration and invasion of bladder cancer cells through the Sonic hedgehog signaling pathway and enhances cisplatin resistance. Biochem Cell Biol. 2022;100:179-87.
- [CrossRef] [Google Scholar]
- The expression and clinical significance of an Epithelial-Mesenchymal transition inducer, SNAI1, in head and neck carcinoma. J Oral Pathol Med. 2021;50:145-54.
- [CrossRef] [PubMed] [Google Scholar]
- NSUN2 Promotes tumor progression and regulates immune infiltration in nasopharyngeal carcinoma. Front Oncol. 2022;12:788801.
- [CrossRef] [PubMed] [Google Scholar]
- SSTR2 in nasopharyngeal carcinoma: Relationship with latent EBV infection and potential as a therapeutic target. Cancers (Basel). 2021;13:4944.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapy for the treatment of advanced nasopharyngeal carcinoma: A promising new era. J Cancer Res Clin Oncol. 2023;149:2071-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunotherapeutic approaches in nasopharyngeal carcinoma. Expert Opin Biol Ther. 2019;19:1165-72.
- [CrossRef] [PubMed] [Google Scholar]
- The role of pyroptosis in modulating the tumor immune microenvironment. Biomark Res. 2022;10:45.
- [CrossRef] [PubMed] [Google Scholar]
- Role and potential of different T helper cell subsets in adoptive cell therapy. Cancers (Basel). 2023;15:1650.
- [CrossRef] [PubMed] [Google Scholar]
- Acute respiratory distress syndrome enhances tumor metastasis into lungs: Role of BRD4 in the tumor microenvironment. Int Immunopharmacol. 2023;115:109701.
- [CrossRef] [PubMed] [Google Scholar]
- Protein C receptor maintains cancer stem cell properties via activating lipid synthesis in nasopharyngeal carcinoma. Signal Transduct Target Ther. 2022;7:46.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-derived extracellular vesicles inhibit ferroptosis and promote distant metastasis of nasopharyngeal carcinoma by upregulating ITGB3. Int J Biol Sci. 2022;18:5858-72.
- [CrossRef] [PubMed] [Google Scholar]
- BRD4 Promotes gastric cancer progression and metastasis through acetylation-dependent stabilization of snail. Cancer Res. 2019;79:4869-81.
- [CrossRef] [PubMed] [Google Scholar]
- Circular RNA circBCBM1 promotes breast cancer brain metastasis by modulating miR-125a/BRD4 axis. Int J Biol Sci. 2021;17:3104-17.
- [CrossRef] [PubMed] [Google Scholar]
- Leveraging big data of immune checkpoint blockade response identifies novel potential targets. Ann Oncol. 2022;33:1304-17.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic assessment of transcriptomic biomarkers for immune checkpoint blockade response in cancer immunotherapy. Cancers (Basel). 2021;13:1639.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic role of programmed cell death ligand-1 expression in head and neck cancer treated with programmed cell death protein-1/programmed cell death ligand-1 inhibitors: A meta-analysis based on clinical trials. J Cancer Res Ther. 2021;17:676-87.
- [CrossRef] [PubMed] [Google Scholar]
- Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 2015;32:86.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations, cancer and the telomere length paradox. Trends Cancer. 2017;3:253-8.
- [CrossRef] [PubMed] [Google Scholar]
- TIPE1-mediated autophagy suppression promotes nasopharyngeal carcinoma cell proliferation via the AMPK/mTOR signalling pathway. J Cell Mol Med. 2020;24:9135-44.
- [CrossRef] [PubMed] [Google Scholar]
- EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14:e1007484.
- [CrossRef] [PubMed] [Google Scholar]
- Adiponectin suppresses tumor growth of nasopharyngeal carcinoma through activating AMPK signaling pathway. J Transl Med. 2022;20:89.
- [CrossRef] [PubMed] [Google Scholar]
- Integrative analysis identified CD38 As a key node that correlates highly with immunophenotype, chemoradiotherapy resistance, and prognosis of head and neck cancer. J Cancer. 2023;14:72-87.
- [CrossRef] [PubMed] [Google Scholar]
- The role of different immunocompetent cell populations in the pathogenesis of head and neck cancer-regulatory mechanisms of pro-and anti-cancer activity and their impact on immunotherapy. Cancers (Basel). 2023;15:1642.
- [CrossRef] [PubMed] [Google Scholar]
- Acylglycerol kinase promotes paclitaxel resistance in nasopharyngeal carcinoma cells by regulating FOXM1 via the JAK2/STAT3 pathway. Cytokine. 2021;148:155595.
- [CrossRef] [PubMed] [Google Scholar]
- Induction chemotherapy with paclitaxel, carboplatin and cetuximab for locoregionally advanced nasopharyngeal carcinoma: A single-center, retrospective study. Front Oncol. 2022;12:951387.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor volume reduction after induction chemotherapy with gemcitabine plus cisplatin in nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2023;280:2497-509.
- [CrossRef] [PubMed] [Google Scholar]
- Exosomes derived from Taxol-resistant nasopharyngeal carcinoma (NPC) cells transferred DDX53 to NPC cells and promoted cancer resistance to Taxol. Eur Rev Med Pharmacol Sci. 2021;25:127-38.
- [Google Scholar]
- Implication of hsa_circ_0028007 in reinforcing migration, invasion, and chemo-tolerance of nasopharyngeal carcinoma cells. J Clin Lab Anal. 2020;34:e23409.
- [CrossRef] [PubMed] [Google Scholar]
- PDK1-and PDK2-mediated metabolic reprogramming contributes to the TGFβ1-promoted stem-like properties in head and neck cancer. Cancer Metab. 2022;10:23.
- [CrossRef] [PubMed] [Google Scholar]






