Translate this page into:
Expression of PD-L1 in Lung Carcinoma and Its Correlation with Histopathological Grade, Stage, and Survival of Patients
Address for correspondence: Vishesh Dhawan, MD, Department of Pathology, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Swami Ram Nagar, Doiwala, Dehradun, 248140, Uttarakhand, India (e-mail: drvishesh93@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
PD-L1, a 40 kDa type 1 transmembrane protein, suppresses the adaptive arm of the immune system. The interaction of PD-1 with the ligand PD-L1 inhibits cytokine production and plays a role in the progression of lung cancer. The present study was performed to observe the expression of PD-L1 in lung carcinoma patients and its correlation with histopathological grade, stage, and survival of patients.
Materials and Methods
This prospective study included all new cases of lung carcinoma diagnosed on histopathological or cytopathological examination over a period of 1 year. PD-L1 immunoexpression was statistically analyzed and graded according to the Tumor Proportion Score in all cases and correlated with histopathological grade, stage, and survival of patients.
Results
This study included 56 cases of lung carcinoma with 64.2% cases showing PD-L1 positivity, out of which 44.6% were non-small cell and 19.6% were small cell lung carcinoma. In all, 32.1% cases with lymphovascular invasion, 53.5% with necrosis, and 37.5% cases with greater than 5/10 HPF mitotic figures showed positive PD-L1 expression. Paired cell blocks and histopathology showed 70% concordance for PD-L1 expression. 16.1% cT3N1M0 cases and 25% stage IIIA cases showed PD-L1 positivity. In all, 60.7% patients with positive PD-L1 expression did not survive for 12 months following diagnosis.
Conclusion
PD-L1 immunoexpression was increased in lung carcinoma cases and was associated with poor histomorphological features including lymphovascular invasion, necrosis, and increased mitotic activity. PD-L1 correlated with cases having decreased 12-month survival and stage IIIA carcinoma. Thus, it may be useful in the stratification of patients who benefit from the PD-L1 targeted therapy.
Keywords
programmed death ligand-1
lung carcinoma
histopathological grade
Introduction
Lung cancer is considered to be one of the most common cancers worldwide. As per GLOBOCAN 2020, the global incidence of lung cancer is 11.4% with 14.3% occurring in males and 8.4% occurring in females.[1] The mortality rate of lung carcinoma is 18% worldwide with 21.5% in males and 13.7% in females. The prognosis of lung cancer depends on various factors including age, gender of the patient, occupation, family history, time of diagnosis, tumor size, site, socioeconomic status, type of lung carcinoma, genetic factors, smoking history with duration and type of smoking. Molecular-targeted therapies have been developed due to the identification of various genetic alterations in lung cancer patients.[2] Programmed death-ligand 1 (PD-L1, B7-H1), a transmembrane protein that is present on antigen presenting cells, myeloid dendritic cells, activated monocytes and B cells, plays an important role in dampening the adaptive arm of the immune system during pregnancy, tissue allografts, autoimmune disease, and disease states such as hepatitis.[3] T cell anergy is induced by tumor, and recognition of tumor antigens by antigen-presenting cells are avoided, a key mechanism by which immune activity is inhibited due to PD-L1 expression by tumor cells.[3] The present study was therefore conducted to analyze PD-L1 immunoexpression and its association with various clinico-pathological parameters in lung carcinoma.
Materials and Methods
This prospective study included newly diagnosed cases of lung carcinoma diagnosed in the pathology department on histopathological or cytopathological examination, over a period of 1 year. Tiny tissue biopsies and cell blocks with less than 20 malignant cells and cytological smears with less than five clusters of six to eight epithelial cells or containing only necrosis, hemorrhagic or mucoid material were excluded from the study. All the relevant clinical and radiological details were recorded for every case. The lung tumors were classified according to the WHO classification of tumors.[4] Immunohistochemical analysis for PD-L1 was performed on paraffin embedded, formalin-fixed sections. RTU mouse monoclonal PD-L1 antibody marker with clone 405.9A11 isotype IgG1, Kappa of BIOGENIX Company was used along with placenta specimen as the control. Cell membrane-positive PD-L1 was graded according to the Tumor Proportion Score (TPS), in which the number of positive tumor cells were divided by the total number of viable tumor cells and then multiplied by 100%. Further, PD-L1 was graded as negative less than 1%, low grade 1–49%, and high grade at 50% or higher, and statistically analyzed.[5] All data were compiled in an Excel sheet and statistically analyzed using the IBM SPSS software version 28 (Statistical Package for Social Sciences) Chicago, USA. The association between categorical variables was studied using Student's t-test and Pearson's correlation coefficient. A p-value less than 0.05 was considered statistically significant. Each patient was closely followed until 1 year with monthly inquiries, either on their hospital visit for therapy or if not, their records confirmed telephonically. Parameters such as hemoptysis, shortness of breath, loss of weight, and decreased appetite, among any other chief complaints, were asked from patients individually. Reports of HRCT, initially done at 3 months following the diagnosis succeeded by three monthly chest X-rays, were used to keep a track on the progression of disease. In cases receiving chemo and radiotherapy, investigations such as complete hemogram, serum creatinine, and liver function tests were also done. The study was approved by the institutional research committee vide letter no. SRHU/HIMS/RC/2022/90.
Results
The study included 56 cases of lung carcinoma with a male to female ratio of 6:1. The mean age of the patients was 62.64 ± 10.48 years, range of 30 to 82 years and median of 62.50 years. The majority of the cases were illiterate (35.7%), belonging to low and middle socioeconomic group (48.2%), non-vegetarians (51.8%), and farmers by occupation (44.6%). Positive PD-L1 expression was observed in 64.2% cases, out of which 44.6% were non-small cell lung carcinoma cases and 19.6% were small cell lung carcinoma cases. PD-L1 positivity was observed in 62.5% smokers and 24% positive PD-L1 cases were smoking for a duration of 20 to 29 years. ►Table 1 shows PD-L1 expression according to different histopathological types of lung carcinoma cases. It was observed that 23.2% squamous cell carcinoma cases, 19.6% adenocarcinoma cases, and 19.6% small cell carcinoma cases showed positive PD-L1 expression (►Fig. 1). ►Table 2 shows an association of histomorphological features with PD-L1 expression. It was observed that 37.5% cases having greater than 5/10 HPF mitotic figures showed a statistically significant different PD-L1 expression (p < 0.05). ►Table 3 shows PD-L1 expression with the clinical stage of lung carcinoma cases. It shows that 25% cases of stage IIIA showed a positive PD-L1 expression with 16.1% cases belonging to c T3N1M0 category. In all, 60.7% patients with lung carcinoma showing positive PD-L1 expression did not survive for 12 months following diagnosis. In all, 20 cases were PD-L1 negative, out of which 19 did not survive for period of 12 months following diagnosis. Paired cell block and histopathological sections observed 70% concordance for PD-L1 immunoexpression (►Fig. 2). One case was inadequate on histopathology but was diagnosed on cytopathology. Amongst the PD-L1-positive lung cancer tissue, ►Fig. 3 depicts a dot plot for lung carcinoma cases showing low-grade PD-L1 positivity.

- Histopathological sections show (A) negative, (B) low, and (C) high PD-L1 expression on squamous cell carcinoma; (D) negative, (E) low, and (F) high PD-L1 expression on adenocarcinoma; (G) negative, (H) low, and (I) high PD-L1 expression on small cell carcinoma (IHC; ×40).

- Comparison of (A) cell block section and (D) histopathological section showing negative PD-L1 expression on squamous cell carcinoma; (B) cell block section and (E) histopathological section showing low PD-L1 expression on adenocarcinoma; (C) cell block section and (F) histopathological section showing high PD-L1 expression on squamous cell carcinoma (IHC; ×40).
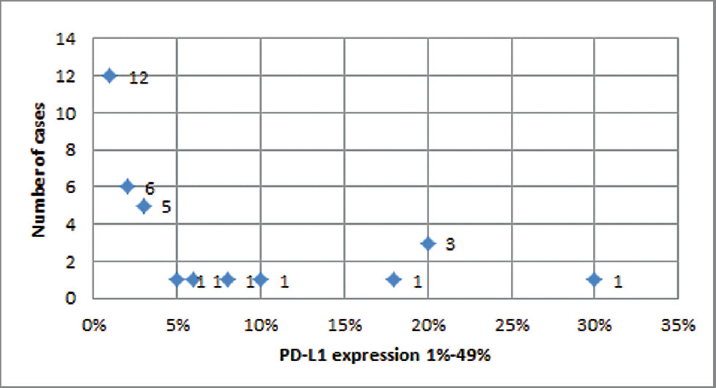
- Dot plot for lung carcinoma cases showing low grade (1% to 49%) PD-L1 positivity.
| Histopathological types | < 1% (Negative) | 1–49% (Low) | ≥ 50% (High) | |
|---|---|---|---|---|
| Squamous cell carcinoma (n = 21) | Moderately differentiated (Grade II) | 5 (23.8%) | 7 (33.3%) | 1 (4.8%) |
| Poorly differentiated (Grade III) | 3 (14.3%) | 5 (23.8%) | 0 (0%) | |
| Adenocarcinoma (n = 18) | Moderately differentiated (Grade II) | 1 (5.6%) | 2 (11.1%) | 0 (0%) |
| Poorly differentiated (Grade III) | 4 (22.2%) | 8 (44.4%) | 1 (5.6%) | |
| Adenocarcinoma NOS | 2 (11.1%) | 0 (0%) | 0 (0%) | |
| Large cell carcinoma (n = 1) | 0 (0%) | 0 (0%) | 1 (100%) | |
| Non-small cell lung carcinoma – NOS (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) | |
| Small cell carcinoma (n = 15) | 4 (26.7%) | 10 (66.7%) | 1 (6.6%) | |
| Histomorphological Features | < 1% (Negative) | 1–49% (Low) | ≥ 50% (High) | Χ2; p-Value | |
|---|---|---|---|---|---|
| Lymphovascular invasion | Present | 6 (10.7%) | 16 (28.6%) | 2 (3.6%) | 2.19; 0.33 |
| Absent | 14 (25%) | 16 (28.6%) | 2 (3.6%) | ||
| Perineural invasion | Present | 0 (0%) | 0 (0%) | 1 (1.8%) | 5.86; 0.07 |
| Absent | 20 (35.7%) | 32 (57.1%) | 3 (5.4%) | ||
| Necrosis | Present | 13 (23.2%) | 27 (48.2%) | 3 (5.4%) | 5.68; 0.05 |
| Absent | 7 (12.5%) | 5 (8.9%) | 1 (1.8%) | ||
| Mitotic figures | < 5/10 HPF | 15 (26.8%) | 14 (25%) | 1 (1.8%) | 6.13; 0.04 |
| > 5/10 HPF | 5 (8.9%) | 18 (32.1%) | 3 (5.4%) | ||
| Inflammation | Absent | 8 (14.3%) | 13 (23.2%) | 2 (3.6%) | 10.36; 0.16 |
| Mild | 5 (8.9%) | 2 (3.6%) | 0 (0%) | ||
| Moderate | 6 (10.7%) | 11 (19.6%) | 0 (0%) | ||
| Dense | 0 (0%) | 4 (7.1%) | 1 (1.8%) | ||
| Mixed | 1 (1.8%) | 2 (3.6%) | 1 (1.8%) | ||
| Stage | < 1% (Negative) | 1–49% (Low) | ≥ 50% (High) | Χ2; p-Value |
|---|---|---|---|---|
| IA | 4 (7.1%) | 3 (5.4%) | 0 (0%) | 14.13; 0.22 |
| IB | 3 (5.4%) | 3 (5.4%) | 0 (0%) | |
| IIA | 0 (0%) | 1 (1.8%) | 0 (0%) | |
| IIB | 0 (0%) | 2 (3.6%) | 0 (0%) | |
| IIIA | 2 (3.6%) | 13 (23.2%) | 1 (1.8%) | |
| IIIB | 1 (1.8%) | 1 (1.8%) | 1 (1.8%) | |
| IVA | 10 (17.9%) | 9 (16.1%) | 2 (3.6%) | |
| Total | 20 (35.7%) | 32 (57.2%) | 4 (7.1%) |
Discussion
Recent studies revealed that lung tumors develop multiple pathways to escape immune system so that they can develop and progress. Prolonged T cell receptor (TCR) stimulation causes an upregulation of PD-L1 expression. PD-L1 is expressed by tumor cells as a consequence of inflammatory cytokines and oncogenic signaling pathways, thus inhibiting TCR-mediated positive signaling, resulting in decreased proliferation, reduced cytokine secretion, and reduced survival of T cells, hence escaping the immune response.[6]
The present study observed that positive PD-L1 expression was seen in 64.2% cases of lung carcinoma. It was observed that cases of non-small cell lung carcinoma and small cell lung carcinoma showed positive PD-L1 expression among males, 60 to 69 years of age group, and having smoking habit for a duration of 20 to 29 years. It was also observed in the present study that PD-L1 was associated with poor histomorphological features such as mitotic figures, lymphovascular emboli, perineural invasion, necrosis, and inflammation although the association was statistically significant with increased mitotic activity. Previous study observed that 53.2% membranous PD-L1 positivity[7] in cases of non-small cell lung carcinoma, which was found in concordance to our study. Studies have also observed that lymphovascular invasion, perineural invasion, necrosis, mitotic figures, and inflammation show association with PD-L1 expression.[7]
The present study observed 16.1% cases of cT3N1M0 and 25% cases of stage IIIA showed positive PD-L1 expression, thus pointing that a high PD-L1 expression was associated with poor survival among the patients. In addition, 60.7% patients of lung carcinoma showing positive PD-L1 expression did not survive for 12 months following diagnosis, suggesting that PD-L1 expression is associated with poorer prognosis among lung cancer patients. The literature search shows that the expression of PD-L1 is associated with increased tumor proliferation and aggressiveness as well as shorter patient survival.[8] Jain et al observed a high PD-L1 expression in 28.2% stage IV tumors.[7] The role of PD-L1 in prognosis of lung carcinoma may be useful in stratifying patients who benefit from PD-L1 pathway-targeted therapy. The National Comprehensive Cancer Network guidelines also point to pembrolizumab therapy that was selected due to PD-L1 IHC testing in all advanced (stage IIIB and IV) lung squamous cell carcinomas and adenocarcinomas.[9]
The present study showed a 70% concordance between cell blocks and paired histopathological sections. Jain et al have also observed a concordance of 88.4% between liquid-based cytology and matched small biopsies, highlighting that liquid-based cytology smears represent a potential resource for immunocytochemistry.[10] Thus, cell blocks may be used as surrogate for PD-L1 expression in lung carcinoma and may be useful in determining prognosis of lung carcinoma.
In our present study, both non-small cell and small cell lung carcinoma cases were included in this study and PD-L1 was analyzed on both histopathological and paired cytopathological (cell blocks) specimens. One case that was found to be inadequate on histopathology was finally diagnosed on cytopathology, highlighting the role of cytopathology as well. Patients were followed up for a duration of 1 year and the survival of each patient was noted. An important limitation of the present study is that lesser number of cases was included with limited time of follow up, which may have resulted in a statistical bias to determine the exact association between PD-L1 expression and lung carcinoma. It is therefore suggested that further larger studies with extended follow-ups may be done to evaluate the exact role of PD-L1 in prognosis of lung carcinoma that may be useful in stratifying patients who benefit from PD-L1 pathway-targeted therapy.
Conclusion
PD-L1 expression was positive in 64.2% of lung carcinoma cases with increased expression in cases associated with the sixth decade of life, male gender, and smokers with a habit of smoking for 20 to 29 years, moderately differentiated squamous cell carcinoma, poorly differentiated adenocarcinoma, and increased mitotic activity. PD-L1 expression was also increased in 60.7% of cases showing decreased 12-month survival, with cT3N1M0 and stage IIIA, suggesting its increased immunoexpression with poor prognosis. It is suggested that for better evaluation of the exact role of PD-L1 in prognosis of lung carcinoma, larger studies with extended follow-ups may be done and thus patients should be stratified who benefit from the PD-L1 pathway-targeted therapy.
Authors' Contributions
V.D. contributed to data curation. V.D. and S.C. contributed to the conceptualization and drafting of the manuscript. S.C., M.K., and S.K. contributed to critical and intellectual evaluation. All authors approve the final manuscript.
Conflict of Interest
None declared.
Funding
None.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(03):209-249.
- [Google Scholar]
- Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol. 2017;7:193.
- [CrossRef] [PubMed] [Google Scholar]
- The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(04):252-264.
- [CrossRef] [PubMed] [Google Scholar]
- WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(09):1243-1260.
- [Google Scholar]
- Role of PD-L1 expression in non-small cell lung cancer and their prognostic significance according to clinicopathological factors and diagnostic markers. Int J Mol Sci. 2019;20(04):824.
- [CrossRef] [PubMed] [Google Scholar]
- CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(01):98-106.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression and its clinicopathologic and genomic correlation in the non-small cell lung carcinoma patients: an Indian perspective. Pathol Res Pract. 2021;228 153497
- [CrossRef] [PubMed] [Google Scholar]
- Impact of smoking on efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer patients: a meta-analysis. OncoTargets Ther. 2018;11:3691-3696.
- [CrossRef] [PubMed] [Google Scholar]
- Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(04):504-535.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death-ligand 1 immunoexpression in matched biopsy and liquid-based cytology samples of advanced stage non-small cell lung carcinomas. Cytopathology. 2018;29(06):550-557.
- [CrossRef] [PubMed] [Google Scholar]






