Translate this page into:
Expression of PD-L1 in malignant soft tissue neoplasm and their correlation with clinicopathological parameters
*Corresponding author: Anurag Singh, MD, Department of Pathology, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India. anugsvm@yahoo.com
How to cite this article: Mishra A, Singh A, Kumar M, Sagar M, Kumari M, Qayoom S, et al. Expression of pd-l1 in malignant soft tissue neoplasm and their correlation with clinicopathological parameters. J Lab Physicians. 2024;16:146-152. doi: 10.1055/s-0043-1771241
Abstract
Objectives:
Programmed death ligand-1 (PD-L1) expression in malignant epithelial neoplasms has been the subject of numerous studies; however, less data on its application to sarcomas are available. This research focused on the expression of PD-L1 and how it correlated with clinicopathological characteristics in soft tissue sarcomas.
Materials and Methods:
The anti-PD-L1 antibody and Ki-67 were stained in 50 cases of sarcoma that had been confirmed by biopsy and immunohistochemistry. The tumor cell percentage with complete or incomplete membrane staining was calculated. Sarcomas were categorized as positive (>1% of tumor cells with complete or incomplete membrane staining) or negative (≤1% of tumor cells with complete or incomplete membrane staining). The data were analyzed using Statistical Package for Social Sciences version 21.0.
Statistical Analysis:
Data were analyzed using Statistical Package for Social Sciences (SPSS, IBM Inc., United States), version 21.0. The independent sample t-test for continuous variables and the chi-square test for discrete variables were used to investigate the relationships between PD-L1 expression and clinicopathologic factors. A p-value less than 0.05 was considered statistically significant.
Results:
The soft tissue sarcomas showing marked pleomorphic morphology were significantly linked to positive PD-L1 expression than other subtypes of sarcomas (p = 0.042). Proliferation index grade III accounts for 62.5% of cases with positive PD-L1 expression, followed by proliferation index grade II with 25% cases and grade I with 12.5% cases. On comparing statistically, this difference was found to be significant (p = 0.013). A significant association was found between PD-L1 expression and the poor outcome of follow-up (p = 0.024).
Conclusions:
Our study showed a significant relationship between malignant soft tissue tumor positivity for PD-L1 and pleomorphic morphology, a higher proliferation index grade, and a poorer prognosis.
Keywords
PD-L1
Immunohistochemistry
Sarcomas
Immunotherapy
immune checkpoint inhibitors
INTRODUCTION
Soft tissue tumors represent complex and heterogeneous mesenchymal neoplasms with a varied range of differentiation. Histopathological classification based on morphological findings often demonstrates the differentiation of specific cell lines. Soft tissue sarcomas account for less than 1% of the overall cancer burden, in contrast to carcinomas and other malignant neoplasms.[1] Immune checkpoint inhibitors have shown great promise in the management of a number of malignancies, including melanoma, non-small cell lung carcinoma, renal cell carcinoma, and soft tissue sarcoma. These agents target the anti-programmed death-1 (PD-1) receptor and the anti-programmed death ligand-1 (PD-L1).[2-4] While PD-L1-targeted treatments have acquired universal recognition for the management of non-small cell lung carcinoma and melanoma, their utility in soft tissue sarcoma is not well established.[5-8]
Advance sarcoma management is challenging, and anti-immune checkpoint inhibitors have been explored as promising therapeutic strategies. The expression of PD-1 or PD-L1 is typically necessary for PD-L1-targeted treatments to be effective. The expression of PDL-1 in various histological types of sarcomas has been examined in a few studies, although the results are variable. The presence of PD-L1 expression may also have important prognostic consequences.[9-12] There are numerous studies on PD-L1 expression in malignant epithelial neoplasms, but less data are available on its utility in soft tissue sarcomas.
Aims and Objectives
In the present research, we studied the immunohistochemical expression of PD-L1 and the Ki-67 proliferation index in various soft tissue sarcomas and their correlation with clinicopathological parameters.
MATERIALS AND METHODS
This was a prospective study done for the duration of 1 year (November 2019–October 2020) in the Department of Pa-thology, King George’s Medical University, Lucknow, in collaboration with the Department of Surgical Oncology, King George’s Medical University, Lucknow, after getting approval from the Institutional Ethical Committee. All the biopsyand immunohistochemistry-proven cases of malignant soft tissue neoplasms were included in the study. The cases with inadequate biopsy tissue for the application of immunohistochemistry were excluded from the study. Patients who did not provide consent were also excluded from the study population.
The final study included 50 cases of malignant soft tissue neoplasms. For each case, clinical details were recorded. PD-L1 and Ki-67 immunohistochemistry were applied and assessed using standard protocols in each case, in addition to histopathological examination of hematoxylin– eosin (H-E)-stained sections. All the cases of malignant soft tissue neoplasms were morphologically graded as per the French Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system. Immunohistochemical staining for PD-L1 and Ki-67 was done on representative sections using the antibody clones E1L3N (Rabbit monoclonal, Ventana, Tucson, Arizona, United States) and DAKO (DSS Imagetech), respectively.
Normal human placental tissue was used as a positive control for PD-L1 and a normal lymph node for the Ki-67 antibody as per the manufacturer’s instructions. The tumor cell percentage with complete or incomplete membrane staining was noted. Sarcomas were categorized as positive (>1% of tumor cells with membrane staining) or negative (≤1% of tumor cells with membrane staining).[13,14] Ki-67 proliferation index was calculated, and grading was done as grade I (0–9% tumor cells with nuclear positivity), grade II (10–29% tumor cells with nuclear positivity), and grade III (2:30% tumor cells with nuclear positivity).[15] In each case, PDL1 expression was accessed by two histopathologists with good experience in regular soft tissue reporting. To reduce the subjectivity both the histopathologists were blinded to clinical details and findings of each other results. The pathologists evaluated and discussed cases where there was a disparity until an agreement was achieved.
Statistical Analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS, IBM Inc., United States), version 21.0. The independent sample t-test for continuous variables and the chi-square test for discrete variables were used to investigate the relationships between PD-L1 expression and clinicopathologic factors. A p-value less than 0.05 was considered statistically significant.
RESULTS
Demographic Profile of the Study Population
The final study population included 50 cases of soft tissue sarcomas. The age range of cases enrolled in the study was from 2 to 70 years. The mean age of the study population was 34.26 ± 18.46 years. The vast majority of the patients (64%) (32/50) were between the ages of 21 and 60 years, followed by 30% (15/50) younger than 20 years, and 6.0% (3/50) of cases were aged >60 years. The majority of cases enrolled in the study were males: 68% (34/50) and 32% (16/50) were females. The male-to-female ratio was 2.13:1.
Distribution of the Study Population According to Histological Grade, Proliferation Index Grade, and Sarcomasubtypes
Among the study population, 52% (26/50) of patients were in FNCLCC grade II, followed by 30% (15/50) in grade III and 18% (9/50) patients in grade I; 38% (19/50) of patients were in proliferation index grade II, followed by 34% (17/50) in grade III and 28% (14/50) patients in grade I. Among soft tissue malignant neoplasms, 36% (18/50) cases were of synovial sarcoma, followed by 18% (9/50) cases of Ewing’s sarcoma, 12% (6/50) of dermatofibrosarcoma protuberans (DFSP), 10% (5/50) liposarcoma, 10% (5/50) leiomyosarcoma, 8% (4/50) undifferentiated pleomorphic sarcoma and 6% (3/50) of rhabdomyosarcoma.
Association of PD-L1 Expression with Proliferation Index Grade
Proliferation index grade III accounts for 62.5% (10/16) of cases with positive PD-L1 expression, followed by proliferation index grade II with 25% (4/16) cases and grade I with 12.5% (2/16) cases. On comparing statistically, this difference was found to be significant (p = 0.013) (Table 1).
| Proliferation index grade | PD-L1 ≤ 1% (n = 34) | PD-L1 >1%(n = 16) | ||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Grade I (n = 14) | 12 | 35.29 | 2 | 12.5 |
| Grade II (n = 19) | 15 | 44.12 | 4 | 25.0 |
| Grade III (n = 17) | 7 | 20.59 | 10 | 62.5 |
Abbreviation: PD-L1, programmed death ligand-1. Chi-square = 8.686 (df = 2); p = 0.013.
Association of PD-L1 Immunohistochemical Expression with Histological Grading, Histopathological Diagnosis, and Prognosis
The majority (56.25%) (9/16) of cases with positive PDL1 expression fall into FNCLCC grade II, followed by 25% (4/16) cases in grade III and 18.75% (3/16) cases in grade I. On comparing statistically, FNCLCC grade did not show a significant association with expression of PD-L1 (p = 0.867) (Table 2).
| Histopathological grading | PD-L1 ≤ 1% (n = 34) | PD-L1 >1% (n = 16) | ||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Grade I (n = 9) | 6 | 17.65 | 3 | 18.75 |
| Grade II (n = 26) | 17 | 50.0 | 9 | 56.25 |
| Grade III (n = 15) | 11 | 32.35 | 4 | 25.0 |
Abbreviation: PD-L1, programmed death ligand-1. Chi-square = 0.285 (df = 2); p = 0.867.
The positive expression of PD-L1 was shown in 75% (3/4) cases of undifferentiated pleomorphic sarcoma, followed by 66.67% (2/3) cases of rhabdomyosarcoma, 60.0% (3/5) cases of liposarcoma, 60.0% (3/5) cases of leiomyosarcoma, 16.67% (3/18) cases of synovial sarcoma, 16.67% (1/6) cases of dermatofibrosarcoma protruberens, and 11.11% (1/9) cases of Ewing’s sarcoma. Out of the total 50 cases, 32% (16/50) were positive for PD-L1 expression, and the proportion of expression of PD-L1 was statistically significant in the different histological subtypes of soft tissue sarcomas (p = 0.042) (Table 3) (Figures 1 and 2).

- Photomicrograph of histopathology section and expression of PD-L1 immunohistochemistry in soft tissue sarcomas. (A) Synovial sarcoma; (B) positive expression of PD-L1 in tumor cells of synovial sarcoma (PD-L1 x400); (C) dermatofibrosarcoma protuberans; (D) positive expression of PD-L1 in tumor cells of dermatofibrosarcoma protuberans (PD-L1 x400); (E) Ewing’s sarcoma; (F) positive expression of PD-L1 in tumor cells of Ewing’s sarcoma (PD-L1 x400). PD-L1, programmed death ligand-1.
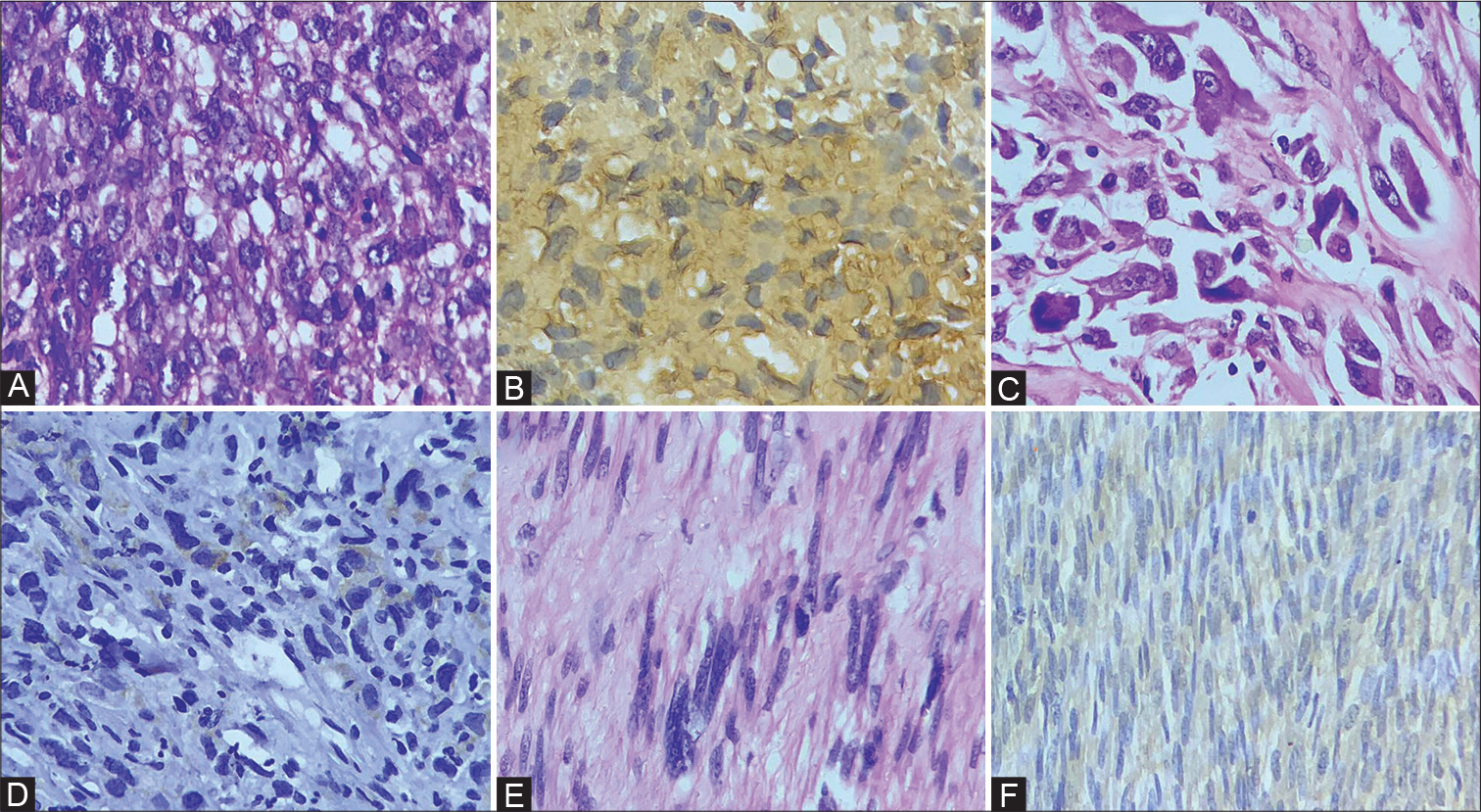
- Photomicrograph of histopathology section and expression of PD-L1 immunohistochemistry in soft tissue sarcomas. (A) Rhabdomyosarcoma; (B) positive expression of PD-L1 in tumor cells of rhabdomyosarcoma (PD-L1 x400); (C) undifferentiated pleomorphic sarcoma; (D) positive expression of PD-L1 in tumor cells of undifferentiated pleomorphic sarcoma (PD-L1 x400); (E) leiomyosarcoma; (F) positive expression of PD-L1 in tumor cells of leiomyosarcoma (PD-L1 x400). PD-L1, programmed death ligand-1.
| Histopathological diagnosis | PD-L1 ≤ 1 (n = 34) | PD-L1 > 1 (n = 16) | ||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Synovial sarcoma (n = 18) | 15 | 83.33 | 3 | 16.67 |
| Dermatofibrosarcoma protuberans (n = 6) | 5 | 83.33 | 1 | 16.67 |
| Ewing’s sarcoma (n = 9) | 8 | 83.89 | 1 | 11.11 |
| Leiomyosarcoma (n = 5) | 2 | 40.0 | 3 | 60.0 |
| Liposarcoma (n = 5) | 2 | 40.0 | 3 | 60.0 |
| Rhabdomyosarcoma (n = 3) | 1 | 33.33 | 2 | 66.67 |
| Undifferentiated pleomorphic sarcoma (n = 4) | 1 | 25.0 | 3 | 75.0 |
Abbreviation: PD-L1, programmed death ligand-1. Chi-square = 13.057 (df = 6); p = 0.042,
Fifty percent (8/16) of patients with positive PD-L1 expression had died, and 31.25% (5/16) patients were alive and doing well during a follow-up period of 1 year; 18.75% (3/16) of patients with positive PD-L1 expression were lost to follow-up. A significant association was found for PDL1 expression with the poorer outcome of follow-up (p = 0.024) (Table 4).
| Follow-up outcome | PD-L1 ≤ 1 (n = 34) | PD-L1 > 1 (n = 16) | ||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Died (n = 13) | 5 | 14.70 | 8 | 50.0 |
| Lost to follow-up (n = 18) | 15 | 44.12 | 3 | 18.75 |
| Alive (n = 19) | 14 | 41.18 | 5 | 31.25 |
Abbreviation: PD-L1, programmed death ligand-1. Chi-square = 7.440 (df = 2); p = 0.024.
DISCUSSION
In the tumor microenvironment, persistent antigen exposure induces the PD-1 receptor, a particular class of inhibitory signal. Tumor cells are able to evade the host immune system due to the interaction between PD-L1 in tumor cells and PD-1 in T lymphocytes.[16] PD-1 and PD-L1 are linked to immune checkpoints that favor tumor escape from immune surveillance. Numerous innovative treatments have been designed that target either PD-1 or PD-L1 in light of a growing understanding of these immune checkpoints. Immunotherapies based on checkpoint inhibition are currently used to treat renal cell carcinoma, non-small cell lung cancer, and melanoma.[17] Immunohistochemical expression of prognostic markers, such as PD-L1 and PD-1, is typically required for the start of therapy with these drugs. The proliferation index (Ki-67) is a nuclear antigen that is used as an immunohistochemical marker to assess the growth percentage in a given cell population, and this protein is seen in all dividing cells.[15,18,19] The Ki-67 proliferation index was used as a prognostic marker that had been investigated in a number of studies for its potential role in breast cancer, soft tissues arcoma, lung cancer, prostate cancer, and cervix carcinoma.[20,21]
Among the study population, 68% (34/50) of patients were male and 32% (16/50) were female. A few prior studies have also found a male majority in their studies.[11,22]
The current study revealed a significant link between positive PD-L1 expression and malignant mesenchymal neoplasms with a more pleomorphic phenotype than other sarcoma subtypes (p = 0.042). The most common soft tissue neoplasm with PD-L1 expression was undifferentiated pleomorphic sarcomas, followed by rhabdomyosarcomas, liposarcomas, leiomyosarcomas, synovial sarcomas, DFSP, and Ewing’s sarcoma. The results of our study are in concordance with previous studies.[23-25] The results of the present study and the literature review could not identify the cause of the variance in PD-L1 expression seen with distinct subtypes of sarcoma. This variation in PD-L1 expression demonstrates the necessity of immunohistochemistry testing to determine whether PD-L1 is present or not before checkpoint inhibitors are used as a treatment.
In our study, 32% (16/50) of the sarcoma cases were PD-L1 positive. The results of the present research are comparable to those from earlier studies by Kim et al and Paydas et al.[11,24]
A few previous studies showed relatively reduced expression of PD-L1 in soft tissue sarcoma cases[13,14,23] (Table 5). Thereported percentages of sarcomas expressing PD-L1 may differ due to sampling size, geographic distribution, the antibodies used, or the cutoff values for positive expression, among possible multifactorial causes.
| Name of author (y) | Number of cases included in study | Criteria for positivity of PD-L1 | Number (%) of PD-L1 positive cases |
|---|---|---|---|
| Orth et al.[14] (2020) | 247 | 1% | 39 (15.8%) |
| Kösemehmetoğlu et al.[23] (2017) | 222 | 5% | 34 (15%) |
| Kim et al.[11] (2016) | 82 | 10% | 35 (43%) |
| Paydas et al.[24] (2016) | 65 | 5% | 19 (29%) |
| D’Angelo et al.[13] (2015) | 50 | 1% | 6 (12%) |
| Present study (Mishra et al., 2023) | 50 | 1% | 16 (32%) |
Abbreviation: PD-L1, programmed death ligand-1.
The correlation between the Ki-67 proliferation index and FNLCC histological grade was statistically significant (p = 0.047) in the present study. The findings of our results were in concordance with previous studies, which also demonstrated the link between high Ki-67 and the histological grade of sarcoma.[26-28]
In the present study, the association between PD-L1 expression and the Ki-67 proliferation index was found to be significant (p = 0.013); 62.50% (10/16) of patients with positive PD-L1 expression had a high proliferation index. Orth et al also observed a significant correlation between PDL1 expression and the tumor cell proliferation index.[14]
PD-L1-positive cases showed a statistically significant (p = 0.024) association with poor prognosis in the present study. Que et al observed in their study that PD-L1 expression was linked with short overall survival (p = 0.001).[22] The results of Kim et al’s and Bertucci et al’s studies are also in concordance with the findings observed in the present study.[11,12]
PD-L1 treatment is known to have a major impact on clinical outcomes for patients with non-small cell lung carcinoma and melanoma, but we lack data from this study to establish what effect it may have on the clinical course of the patients. Based on these findings, PD-L1 therapy for positive expressors may improve patient survival.
The small sample size, an unequal distribution of histologic subtypes of sarcomas, and a brief follow-up period are the main limitations of our study. It is therefore recommended to conduct studies with larger sample sizes and longer follow-up durations.
CONCLUSIONS
Our study revealed a significant correlation between malignant soft tissue tumor positivity for PD-L1 expression and pleomorphic morphology, a higher proliferation index grade, and a poorer prognosis in malignant soft tissue tumors. Our results also demonstrate the necessity of PD-L1 testing before starting PD-L1 checkpoint inhibitor therapy in soft tissue sarcomas. Larger clinical trials and longer follow-up are necessary to determine its function beyond that of a predictive biomarker.
Authors ’ Contribution
A.M. did a literature search, data acquisition, conceptual analysis, and proof correction. A.S. did conceptual analysis, study designing, literature search, and manuscript writing. M.K. did data analysis and designing. M.S. did data analysis, study designing, and proofreading. M.K. did conceptual analysis and data acquisition. S.Q. did literature search and proof reading. V.K. contributed to clinical studies, literature search and critical appraisal.
Ethical Approval
The study is approved by the Research Ethics Committee of King George’s Medical University Lucknow (97th ECM IIBThesis/P37, Letter number 1717/Ethics/19).
Conflict of Interest
None declared.
Fuding
No funding and not a part of the employment of the authors.
References
- The pathology of soft tissue sarcomas. Radiol Med (Torino). 2019;124:266-281.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers. Arch Pathol Lab Med. 2017;141:851-861.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847-856.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death ligand-1 immunohistochemistry: friend or foe? Arch Pathol Lab Med. 2016;140:326-331.
- [CrossRef] [PubMed] [Google Scholar]
- Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist. 2017;22:81-88.
- [CrossRef] [PubMed] [Google Scholar]
- The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992968.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84-106.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression in sarcomas: an immunohistochemical study and review of the literature. Ann Diagn Pathol. 2021;55:151823.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and prognostic implications of PD-L1 expression in soft tissue sarcomas. Pathol Oncol Res. 2021;27:1609804.
- [CrossRef] [PubMed] [Google Scholar]
- Status of programmed death-ligand 1 expression in sarcomas. J Transl Med. 2018;16:303.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16:434.
- [CrossRef] [PubMed] [Google Scholar]
- PDL1 expression is a poorprognosis factor in soft-tissue sarcomas. OncoImmunology. 2017;6:e1278100.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357-365.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative view on the expression patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunology Immunotherapy. 2020;69:1353-1362.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of Ki-67 in prognostication of soft tissue tumors. Ann Pathol Lab Med. 2017;4:A579-A584.
- [CrossRef] [Google Scholar]
- Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2:e000213.
- [CrossRef] [PubMed] [Google Scholar]
- PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561.
- [CrossRef] [PubMed] [Google Scholar]
- The role ofexosomal PDL1 in tumor progression and immunotherapy. Mol Cancer. 2019;18:146.
- [CrossRef] [PubMed] [Google Scholar]
- The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322.
- [CrossRef] [Google Scholar]
- Ki67 is a promising molecular target in the diagnosis of cancer (review) Mol Med Rep. 2015;11:1566-1572.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of p53 and Ki67 expression in fiberoptic bronchial biopsies of patients with non small cell lung cancer. Multidiscip Respir Med. 2012;7:29.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 expression is associated with FOXP3þ regulatory T-cell infiltration of soft tissue sarcoma and poor patient prognosis. J Cancer. 2017;8:2018-2025.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed death ligand 1 (PD-L1) expression in malignant mesenchymal tumors. Turk Patoloji Derg. 2017;1:192-197.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med Oncol. 2016;33:93.
- [CrossRef] [PubMed] [Google Scholar]
- PD-L1 and PD-1 and characterization of tumor-infiltrating lymphocytes in high grade sarcomas of soft tissue prognostic implications and rationale for immunotherapy. OncoImmunology. 2017;7:e1389366.
- [CrossRef] [PubMed] [Google Scholar]
- Ki-67 expression and clinicopathological features of sarcomas. Pan Arab J Oncol. 2019;12:37-42.
- [Google Scholar]
- Prognostic significance of Ki-67 reactivity in soft tissue sarcomas. Cancer. 1989;63:1607-1611.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of Ki-67 expression in 182 soft tissue sarcomas. Proliferation-a marker of metastasis? Acta Pathol Microbiol Scand Suppl. 1994;102:915-924.
- [CrossRef] [PubMed] [Google Scholar]





