Translate this page into:
Histopathological Evaluation of Angiogenic Markers in Non-Hodgkin's Lymphoma
Address for correspondence: Anita Tahlan, MD, DNB, DM, Department of Pathology, Haematology Clinic, Government Medical College and Hospital, Sector-32, Chandigarh, India (e-mail: Anitatahlan@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Angiogenesis plays a key role in the development, maintenance, and progression of tumor. The incidence of non-Hodgkin's lymphoma (NHL) is increasing from the past three decades.
Materials and Methods
The aim of the study is to evaluate microvessel density (MVD) using CD34 monoclonal antibody and vascular endothelial growth factor (VEGF) using monoclonal antibody that were studied in pretreatment paraffin-embedded tissue samples of 60 cases.
Results
MVD was found to be increased in parallel with increasing grade of tumor. B-NHL had a mean MVD of 79.5 ± 8.8 (no./mm2), while T-NHL had a mean MVD of 183 ± 37.6 (no./mm2). VEGF expression was seen in 42 cases (70%), 20 cases (33.3%) showed strong VEGF expression, and the remainder showed either weak (36.6%) or no (30%) staining. Strong VEGF expression is seen in 100% cases of T-NHL and 77.7% cases of B-NHL. Mean MVD and VEGF expression was found to be correlated significantly with the histological grade of NHL (p = 0.001 and p = 0.000, respectively). Average microvessel counts were 53, 82.9, and 130.8 vessels (no./mm2) for negative, weak, and strong VEGF staining, respectively. These differences were statistically significant (p = 0.005 for strong vs. negative and p = 0.091 for strong vs. weak VEGF staining individually).
Conclusion
As the grade of tumor progresses, the angiogenic potential also advances which seems to depend on VEGF. The presence of higher MVD in high-grade lymphomas can be utilized for antiangiogenic drugs.
Keywords
angiogenesis
CD34
hot spots
microvessel density
non-Hodgkin's lymphoma
Introduction
Non-Hodgkin's lymphoma (NHL) is a heterogeneous group of lymphoproliferative neoplasm with different presenting features, clinical course, and response to treatment. It represents the 10th most commonly diagnosed cancer and ranks 7th among developed countries.[1] It is one of the most common cancers in the United States accounting for ∼4% of all cancers.[2] It can be classified depending on the type of white blood cell in which the cancer begins: B cell (∼85%) or T cell.[3] B cell lymphomas are constantly more common around the world, whereas T cell lymphomas are proportionally more common in Asia than in Western countries.[4]
The poor prognostic factors include age (older than 60 years), stage (III or IV disease), elevated serum lactate dehydrogenase, performance status (2, 3, or 4), and location at more than one extranodal site. Other prognostic factors include expression of p53, angiogenic activity, and proliferative activity of NHL.[5]
Angiogenesis is required for tumor growth and metastasis and is an important component in the control of cancer progression.[6] Lymphoma growth and progression are potentiated by at least two distinct angiogenic mechanisms: autocrine stimulation of tumor cells via expression of vascular endothelial growth factor (VEGF) and VEGF receptors on lymphoma cells as well as paracrine influences of proangiogenic tumor microenvironment on both local neovascular transformation and recruitment of circulating bone marrow-derived progenitors. Various growth factors that have been proved to stimulate angiogenesis include fibroblast growth factor, transforming growth factor α, platelet-derived growth factor, and VEGF.[7] VEGF regulates several endothelial cell functions including mitogenesis, permeability, vascular tone, and the production of vasoactive molecules. Neoangiogenesis process in cancer is influenced by the local microenvironment of the tumor itself.[8]
Microvessel density (MVD) measures lymphoma neovascularization, which is generated in response to proangiogenic stromal cells and infiltrating benign T/B lymphocytes within the tumor microenvironment. Microvessels are visualized by immunohistochemical staining, and objective quantification can be done using microscope, but image morphometry has shown better results. In the area showing the most intense vascularization (i.e., the “hot spot”), MVD, total vascular area, as well as the size-related parameters can be estimated by using image analyzer.[9]
Indian studies assessing the expression of angiogenic markers are limited. Therefore, the present study is planned to analyze the expression of angiogenic markers in NHL.
Materials and Methods
Tissue Sampling and Histologic Evaluation
Angiogenesis was measured on 60 cases morphologically diagnosed as NHL from Department of Pathology, Government Medical College and Hospital, Panjab University, Chandigarh, India. Only de novo cases of nodal and extranodal NHLs were included in the study. The specimens were received and fixed in 10% formalin. The specimen was processed by paraffin-embedding technique. Sections were cut at 2 to 3 µ thickness for hematoxylin and eosin (H&E) staining immunostaining. H&E-stained sections were examined. The detailed microscopic examination was done for tumor diagnosis and type. The various immunostains applied are CD3, CD20, CD10, BCL6, BCL2, CD30, CD5, CD23, CyclinD1, ALCL, Ki67, CD4, CD8, CD34 and VEGF.
Immunohistochemistry
Immunohistochemical analysis was performed on paraffin sections using the following antibodies: CD3 (Clone F 7.2.38: DAKO), CD20 (Clone L-26: DAKO), VEGF (VG-1: DAKO), and CD34 (Class II, Clone QBEnd 10, DAKO); CD3 and CD20 immunostaining were done to subtype the NHL into B- or T-NHL. The sections were brought to water, rinsed with distilled water followed by endogenous blocking with 3% H2O2 in methanol. After 10 to 20 minutes, slides were washed with water and then with tris buffer, and antigen retrieval was done by using microwave in citrate buffer (pH 6.0) for 12 to 15 minutes for CD3, CD20, and CD34 and 40 minutes for VEGF. And then, slides were washed with tris buffer. Then, primary antibody was applied in a moisturizing chamber. Slides were then washed with tris buffer. One step Envision (30 minutes) was done and washed with tris buffer. Chromogen (DAB) was applied. Counterstaining was done with Cole's hematoxylin. Results were obtained by studying cells.
The positive internal control sections for CD3 and CD20 was seen in paracortical region and residual germinal center of lymph node, respectively. The positive control sections for CD34 were taken from kidney and spleen, while for VEGF were taken from hemangioma and placenta.
Scoring of Immunohistochemical Stains
Immunohistochemical stains for VEGF were scored as follows based on the percentage of lymphoma cells stained. Cytoplasmic and membrane staining were regarded as positive. The scoring was done; more than 30% staining scored strong positive (++); 5 to 30% staining scored weak positive (+); and less than 5% staining scored negative (−).[10]
Microvessel Density
MVDs were evaluated by counting the number of CD34-positive microvessels by using image analysis program—Proplus 9 on Olympus microscope.
CD34-stained smears were selected from each case that contained hot spots. Hot spots are the areas that showed the most intensive vascularization at ×100 magnification, that is, areas of maximal MVD.
Photomicrographs of the hot spots' area were taken using D20-DRV Version, Olympus Corporation.
The photomicrographs were taken at ×400 magnification.
In three such hot spots in each case, all microvessels (defined as distinct CD34+ cell or cell cluster, irrespective of lumen) were counted at ×400 magnification (each field representing an area of 0.375 mm2).
The total count of microvessels in the hot spot (MVD) and a mean value were taken.
MVD was calculated and expressed as number of microvessels per mm2.
Statistical Analysis
Descriptive statistics were used for statistical analysis of the observed data. Analysis of variance test was applied for the observations of MVD and Pearson's chi-square test for VEGF to find agreement with the final histopathology findings. Mann–Whitney's test was applied to the findings of MVD with histological subtype. Pearson's correlation was used to compare the data.
Results
Clinicopathological Results
The age of the patients ranged from 16 to 97 years with a mean of 55.9 ± 16.9 years with male-to-female ratio of 2.1:1. Maximum number of cases, that is, 17 (28.3%) was in seventh decade. The most common presenting symptom among the patients was unexplained fatigue and weight loss. Clinical staging of patients was 28 (46.7%) patients had stage I, 4 (6.7%) stage II, 7 (11.6%) stage III, and 21 (35%) stage IV.
Twenty-seven cases (45%) presented with the lymphadenopathy, and extranodal presentation was seen in 33 (55%) cases with gastrointestinal tract involvement accounting for 16.6% of all NHL cases and stomach (60.0%) being the most common extranodal presentation. On histological examination, 54 (90%) cases were B-NHL and 6 (10%) were T-NHL. Among B-NHL, 24 (44.4%) cases were low grade, 12 (22.2%) were intermediate grade, and 18 (33.3%) were of high grade[11] (►Table 1).
| Histopathological subtypes | No. of cases (n = 60) | MVD with SE (no./mm2) | VEGF | ||
|---|---|---|---|---|---|
| N | W | S | |||
| B-NHL | 54 | 79.5 ± 8.8 | 18 | 22 | 14 |
| Low grade | 24 | 51.09 ± 8.03 | 18 | 6 | 0 |
| Follicular lymphoma | 4 | ||||
| B cell chronic lymphocytic leukemia/small | 9 | 47.5 ± 8.18 | 6 | 4 | 0 |
| Lymphocytic lymphoma | 38.5 ± 11.2 | 3 | 0 | 0 | |
| Mantle cell lymphoma | 3 | ||||
| Nodal marginal zone B cell lymphoma | 2 | 60 ± 24.7 | 2 | 1 | 0 |
| Splenic marginal zone lymphoma | 1 | 55 ± 8.4 | 2 | 0 | 0 |
| Lymphoplasmacytic lymphoma | 1 | 51 | 1 | 0 | 0 |
| Hairy cell leukemia | 1 | 85 | 1 | 0 | 0 |
| Extranodal marginal zone B cell lymphoma | 3 | 52 | 1 | 0 | 0 |
| MALT type | 70 ± 9.5 | 3 | 1 | 0 | |
| Intermediate grade | 12 | 77.3 ± 10.65 | 0 | 12 | 0 |
| Follicular lymphoma | 12 | 0 | 12 | 0 | |
| High grade | 18 | 117 ± 13.89 | 0 | 4 | 14 |
| Diffuse large B cell lymphoma | 12 | 139 ± 35.8 | 0 | 0 | 12 |
| Burkitt's lymphoma | 1 | 103 | 0 | 0 | 1 |
| Precursor B-acute lymphoblastic | 4 | 69.15 ± 5.9 | 0 | 3 | 1 |
| Lymphoma/leukemia | 0 | 1 | 0 | ||
| Mantle cell lymphoma (blastoid) | 1 | 72 | 0 | 0 | 0 |
| Primary mediastinal B cell lymphoma (high grade) | 0 | 0 | 0 | 0 | 0 |
| T-NHL | 6 | 183 ± 37.6 | 0 | 0 | 6 |
| Hepatosplenic T cell lymphoma | 1 | 167 | 0 | 0 | 1 |
| Anaplastic large cell lymphoma, ALK positive | 2 | 157 ± 36 | 0 | 0 | 2 |
| Peripheral T cell lymphoma, NOS | 2 | 230 ± 13 | 0 | 0 | 2 |
| Angioimmunoblastic T cell lymphoma | 1 | 177 | 0 | 0 | 1 |
Abbreviations: ALK, anaplastic lymphoma kinase; MALT, mucosa-associated lymphoid tissue; MVD, microvessel density; N, negative; NHL, non-Hodgkin's lymphoma; NOS, not otherwise specified; S, strong positive; SE, standard error; VEGF, vascular endothelial growth factor; W, weak positive.
In many cases, cytokeratin, desmin, chromogranin immunostains were done to rule out lymphoma from other epithelial, mesenchymal, and neuroendocrine tumors.
Immunohistochemical Expression of VEGF
Majority of lymphomas, 42 cases (70%) expressed VEGF immunoreactivity, 20 cases (33.3%) showed strong VEGF expression, 22 cases (36.7%) showed weak, and the remainder 18 cases (30%) showed no staining. VEGF expression increases with the advanced grade, that is, among low-grade B-NHL, 75% (18/24) showed no expression, 100% (12/12) intermediate grade showed weak expression, and among high grade, 77.7% (14/18) showed strong expression and strong statistical difference (p = 0.000) (►Figs. 1 and 2).

- Photomicrograph showing (A) neoplastic follicles of low-grade NHL (H&E, ×40), (B) VEGF (×200), and (C) MVD highlighted by CD34 expression (×4,000) in low-grade NHL, (D) high-grade NHL (H&E, ×600), (E) VEGF expression (×600), and (F) MVD by CD34 (×400) in high-grade NHL. H&E, hematoxylin and eosin; MVD, microvessel density; NHL, non-Hodgkin's lymphoma; VEGF, vascular endothelial growth factor.

- Mean MVD and VEGF expression with histological grade of NHL. HG, high grade; IG, intermediate grade; LG, low grade; MVD, microvessel density; NHL, non-Hodgkin's lymphoma; VEGF, vascular endothelial growth factor.
Strong VEGF expression is seen in all cases of T-NHL (100%) and in 14 (77.7%) cases of high-grade B-NHL which was correlated statistically (p = 0.36).
MVD
Maximum number of vessels was counted as 240 in a field that had an area of 0.375 mm2. The mean MVD was 89.9 ± 16.2 (no./mm2) in all patients. Mean MVD was found to be correlated significantly with the morphological grade of NHL (p = 0.001), that is, it was found to be increased in parallel with increasing grade of tumor, that is, high-grade B-NHL had highest MVD with a mean value of 117.4 ± 13.89 (no./mm2) followed by intermediate-grade and low-grade B-NHLs (►Figs. 1 and 2).
T-NHL had a mean MVD of 183 ± 37.6 (no./mm2) in a range of 112 to 240 which was higher as compared with B-NHL and was statistically significant (p = 0.022).
Among B-NHL, small lymphocytic lymphoma has the lowest VEGF and MVD, while diffuse large B-cell lymphoma (DLBCL) has the highest angiogenic potential. Among T-NHL, peripheral T cell lymphomas have the soaring angiogenic capacity.
Average MVDs increase with strength of VEGF staining. Average microvessel counts were 53, 82.9, and 130.8 vessels (no./mm2) for negative, weak, and strong VEGF staining, respectively. These differences were statistically significant (►Fig. 3) (p = 0.005 for strong vs. negative and p = 0.091 for strong vs. weak VEGF staining individually).
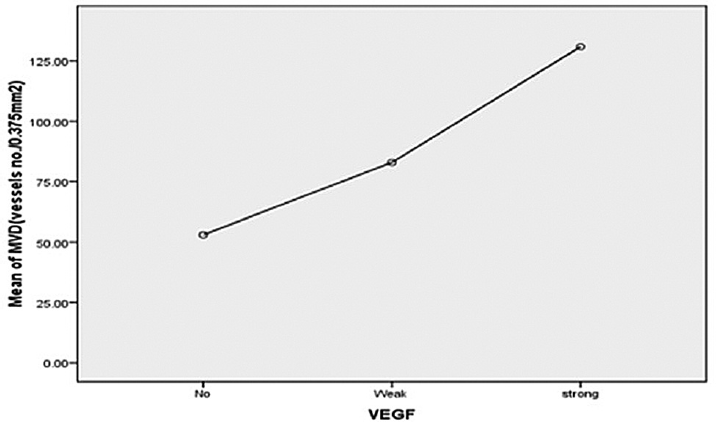
- Correlation between VEGF and mean MVD in NHL (Pearson's correlation coefficient = 0.562, p = 0). MVD, microvessel density; NHL, non-Hodgkin's lymphoma; VEGF, vascular endothelial growth factor.
Discussion
Non-Hodgkin's lymphoma (NHL) includes all lymphomas except for Hodgkin's lymphoma. The incidence of NHL has increased worldwide in the past three decades.[12] It is now the fifth most common malignant neoplasm in the United States, after cancers of the breast, prostate, lung, and colon and eighth most common cancer in the United Kingdom.[13,14] As this disease usually becomes symptomatic at an advanced stage, this malignancy comprises a disproportionate number of cancer deaths.
The age range of the patients was 16 to 97 years with a mean of 55.9 ± 16.9 years. The median age was 60 years. Out of 60 cases, 17 cases were presented in the age range of 61 to 70 years. One-third of B-NHL was in seventh decade. Similar trend is noted in other studies.[15-18] The present study highlights the male predominance 2.1:1 which goes in concordance with other studies.[13,17,19] The nodal and extranodal presentation were seen in 27 (45%) and 33 cases (55%), respectively. Among extranodal lymphomas, gastrointestinal tract involvement was seen in 10 cases representing 16.7% of all NHL cases and most commonly involved site being stomach (60%) followed by large intestine and small intestine (20% each).
These findings are in concordance with other studies done by d'Amore et al,[20] Koch et al,[21] and Papaxoinis et al.[22]
NHL is a diverse group of neoplasm which shows subtle histological differences.[23] In the present study, low-grade lymphomas are the commonest type accounting for 40% of all NHL followed by high-grade lymphomas accounting for 36.7% of all NHL and 23.3% of intermediate-grade NHL. A study done in Turkey by Hazar et al[18] showed predominance of intermediate grade followed by low-grade and high-grade lymphomas, and among 71 cases of NHL, the histologic subtype could not be determined in 10 cases. Another study done by Akhter et al[15] in 50 cases of NHL reported high-grade lymphomas (68%) to be more common than low grade (32%). Many Western studies showed DLBCL (high grade) lymphomas to be the commonest (25–30%).[24,25] Similar trend is noted in Jordan (28.2%) and India.[26,27] This is not in concordance with our study. This may be a reflection of geographic variation of the disease and due to low sample size of the study.
In our study, 90% cases were B-NHL and 10% were T-NHL. Several similar series about the frequency of T cell lymphoma had been reported previously.[28-31] In Bangladesh, one similar study done by Haque[32] found that T-NHL accounts 21.36% which was higher than our study.
Among B-NHL, low-grade lymphomas account for 44.4% followed by high grade accounting for 33.4% and intermediate grade accounting for 22.2%. A study done in Germany by Vacca et al[33] in 71 cases of B-NHL revealed majority of low-grade NHL accounting for 38% followed by 33.8% of high-grade and 28.2% of intermediate-grade lymphomas which is comparable with our study
Angiogenesis plays an important role in development, maintenance, and progression of hematological malignancies. The progression of tumor is characterized by uncontrolled growth of tumor cells. The growth of small tumors is initially limited by distance beyond which nutrients and oxygen can diffuse. Thus, angiogenesis is known to be a prerequisite factor for tumor growth beyond a few (1–3) mm2 in size. MVD using CD34 monoclonal antibody and VEGF using monoclonal antibody for VEGF expression were studied in paraffin-embedded tissue samples of all the 60 cases in the present study.
The present study revealed highly significant increase 117 ± 13.8 and 183 ± 37.6 (no./mm2) in MVD high-grade B-NHL and T-NHL, respectively (►Table 1). This increased MVD in high-grade NHL could be because of hypoxic environment which is produced due to rapidly diving cells and large size of tumor. However, lower MVD of 51.1 ± 8 no./mm2 is seen in low-grade NHL as compared with both high-grade and intermediate-grade NHLs which may be attributable to their least proliferative activity in comparison to both intermediate- and high-grade tumors. So, increase in MVD with increase in the grade of tumor was found to be statistically significant (p-value = 0.001). These results are in agreement with studies done by some authors who have demonstrated increased vascularity in Hodgkin's disease and NHL with higher counts in high-grade lymphomas.[34] Studies exploring angiogenesis by measurement of MVD have shown that MVD scores are the highest in aggressive subtypes including Burkitt's lymphoma and peripheral T-cell lymphoma and the lowest in indolent lymphomas.[35] Arias and Soares[36] found a statistically significant difference in MVD measured by immunostaining with antifactor VIII antibody between low- and high-grade NHLs, when classified in either the Working Formulation or the Kiel classification. Our study showed comparable result with high mean MVD in high-grade NHL than low-grade NHL. There are some other studies which showed similar results.[19]
An overall higher MVD is noted in T-NHL (183 ± 37.6 no./mm2) as compared with B-NHL (79.5 ± 8.8 no./mm2) in the present study which is in agreement with a study suggesting enhanced angiogenesis assessed by MVD in aggressive lymphomas such as peripheral T cell lymphomas (183.42 ± 8.24 per mm2) and diffuse large B cell lymphomas (149.91 ± 13.68 per mm2). On the other hand, indolent lymphomas such as small lymphocytic lymphomas (76.78 ± 10.41 per mm2) and follicular lymphomas (141.21 ± 23.33 per mm2) had significantly lower MVD.[19]
The present study showed strong VEGF expression among high-grade NHL than intermediate- and low-grade lymphomas (►Fig. 2) and found to be statistically significant (p-value = 0.000) which is in concordant with the study done by Gratzinger et al[10] done on 94 cases of DLBCL specimens which showed higher local VEGF expression. Increased level of serum VEGF has been reported more frequently in high-grade NHL.[37–39] Foss et al[40] showed that VEGF was minimally expressed or absent in low-grade B cell lymphomas which is similar to our study. The VEGF expression was analyzed in tissues by employing in situ hybridization with a 35S-labeled RNA probe specific for this cytokine. However, no statistically significant difference was found for VEGF expression between subtypes of B cell NHL, as well as between low- and high-grade groups in a study done by Ozbudak et al[41] in Turkey on 51 patients of B-NHL. These findings are not in agreement with present study. Ganjoo et al[42] reported high VEGF expression to have a negative impact on outcome in 97 cases of DLBCL. However, we were not able to speculate reliably on the results regarding this subject in this study, due to the lack of clinical follow-up data.
A very strong association has been seen between T-NHL and strong VEGF expression in the present study. VEGF expression increases with advanced stage, and this was in agreement with the study by Ribatti et al.[43]
In the present study, higher MVD is present in higher grade specimens expressing higher levels of VEGF. This finding is consistent with a paracrine role of VEGF elaborated by lymphoma cells in tumor angiogenesis. If VEGF immunohistochemistry is reflective of effective local VEGF signaling, and if local VEGF signaling is an important factor in angiogenesis, we would expect MVD to increase with local VEGF expression. Average MVDs did increase with strength of VEGF staining. Average microvessel counts were 53, 82.9, and 130.8 vessels (no./mm2) for negative, weak, and strong VEGF staining, respectively. These differences were statistically significant (►Fig. 3) (p = 0.005 for strong vs. negative and p = 0.091 for strong vs. weak VEGF staining individually). Present study results go in agreement with Gratzinger et al[10] where average microvessel counts were 20, 39, and 51 vessels per 1.0-mm core for negative, weak, and strong VEGF staining, respectively.
Few earlier studies have correlated MVD with expression of angiogenic factors and receptors. In diffuse large B cell lymphomas, the association of MVD with tumor cell expression of VEGF has shown conflicting results in various studies.[10,44] This however does not go in favor of the present study.
Alshenawy[7] showed similar results where average MVD increases with strength of VEGF staining with statistical significance, and this was also demonstrated in a study by Streubel et al.[45] A positive correlation coefficient was observed between VEGF score and MVD in all the hematological malignancies. Similar finding has also been observed by Gianelli et al[46] who concluded that VEGF expression correlates with MVD in Philadelphia chromosome-negative chronic myeloproliferative disorders.
Conclusion
In the present study, the various variables studied are MVD, VEGF, and histopathological parameters. Since the Pearson's correlation is r ≥ 0.562 and corresponding p-value is ≤0.01, angiogenic factors (MVD and VEGF) correlated significantly with histopathological parameters. The Spearman's rho correlation coefficients of all the variables were ≥ 0.570 and showed positive correlation. Hence, angiogenesis in NHL can be accurately measured by MVD using CD34 and VEGF immunostaining. However, a large study with follow-up is further required to provide valuable prognostic and predictive information between angiogenic markers and histopathological parameters.
Conflict of Interest
None declared.
Funding
None.
References
- Epidemiology and etiology of non-Hodgkin lymphoma–a review. Acta Oncol. 2006;45(03):258-271.
- [CrossRef] [PubMed] [Google Scholar]
- Accessed October 14, 2014, at: http://www.cancer.org/acs/groups/cid/document/webcontent/003126pdf.pdf
- Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84(01):1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of Lymphoid Malignancy in Asia, Epidemiology Insights, Dr. Maria De Lourdes Ribeiro De Souza Da Cunha (Ed.), ISBN: 978-953-51-0565-7, InTech. 2012 Available from: http://www.intechopen.com/books/epidemiology-insights/epidemiology-of-lymphoid-malignancy-in-asia
- [CrossRef] [Google Scholar]
- Adult non-Hodgkin lymphoma treatment: cellular classification of adult non-Hodgkin lymphoma. Accessed October 14, 2014, at: http://www.cancer.gov/cancertopics/pdq/treatment/adult-nonhodgkins/HealthProfessional/page2
- [Google Scholar]
- Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6, suppl 16):15-18.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of vascular endothelial growth factor, basic fibroblastic growth factor, and microvessel density and their relation to cell proliferation in B-cell non-Hodgkin's lymphoma. Ann Diagn Pathol. 2010;14(05):321-327.
- [CrossRef] [PubMed] [Google Scholar]
- Angiogenesis and antiangiogenic therapy in non-Hodgkin's lymphoma. Ann Oncol. 2009;20(03):413-424.
- [CrossRef] [PubMed] [Google Scholar]
- Morphometric angiogenesis parameters for indolent and aggressive non-Hodgkin's lymphoma. Med Arh. 2011;65(01):9-12.
- [Google Scholar]
- Microvessel density and expression of vascular endothelial growth factor and its receptors in diffuse large B-cell lymphoma subtypes. Am J Pathol. 2007;170(04):1362-1369.
- [CrossRef] [PubMed] [Google Scholar]
- Non-Hodgkin's lymphomas: current classification and management. CA Cancer J Clin. 1997;47(06):351-372.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94(16):1204-1210.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer surveillance series: non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92(15):1240-1251.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphoma: an introduction into historical background, classification schemes, aetiology, geographical variation and epidemiological trend. Malays J Pathol. 2001;23(02):49-63.
- [Google Scholar]
- Histological Subtypes of Non-Hodgkin's Lymphoma in Different Age and Sex Groups. Bangladesh Medical Journal. 2014;41(01):32-36.
- [CrossRef] [Google Scholar]
- Improved survival for non-Hodgkin lymphoma patients in New South Wales, Australia. BMC Cancer. 2010;10:231.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of non-Hodgkin lymphomas in Tyrol/Austria from 1991 to 2000. J Clin Pathol. 2006;59(01):48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of microvessel density and vascular endothelial growth factor (VEGF) expression in non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44(12):2089-2093.
- [CrossRef] [PubMed] [Google Scholar]
- Angiogenesis in Non-Hodgkin's Lymphoma: An Intercategory Comparison of Microvessel Density. ISRN Hematol. 2012;2012 943089
- [CrossRef] [PubMed] [Google Scholar]
- Danish Lymphoma Study Group. Non-Hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. J Clin Oncol. 1994;12(08):1673-1684.
- [CrossRef] [PubMed] [Google Scholar]
- German Multicenter Study Group. Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001;19(18):3861-3873.
- [CrossRef] [PubMed] [Google Scholar]
- Primary gastrointestinal non-Hodgkin's lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG) Leuk Lymphoma. 2006;47(10):2140-2146.
- [CrossRef] [PubMed] [Google Scholar]
- Histological classification of the non-Hodgkin's lymphoma. Blood Rev. 1990;4(02):111-115.
- [CrossRef] [PubMed] [Google Scholar]
- (Eds) WHO Classification of Tumours. Pathology and Genetics of Tumours of Hematopoietic and Lymphoid Tissues. (4th). Lyon: IARC Press; 2008.
- [Google Scholar]
- Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's lymphoma classification project. Ann Oncol. 1998;9(07):717-720.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant lymphoma in Jordan: a retrospective analysis of 347 cases according to the World Health Organization classification. Ann Saudi Med. 2005;25(05):398-403.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of various subtypes of non-Hodgkin's lymphoma in India: a study of 2773 lymphomas using R.E.A.L. and WHO classifications. Ann Oncol. 2000;11(Suppl 1):63-67.
- [CrossRef] [PubMed] [Google Scholar]
- International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
- [CrossRef] [PubMed] [Google Scholar]
- Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117(03):823-834.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical and other prognostic factors in B cell non Hodgkin lymphoma patients, Kampala, Uganda. BMC Clin Pathol. 2009;9:11-15.
- [CrossRef] [PubMed] [Google Scholar]
- Peripheral T-cell lymphoma in the neck: CT findings of lymph node involvement. AJNR Am J Neuroradiol. 2006;27(05):1079-1082.
- [Google Scholar]
- A Study on Frequency, Histologic Types and Immunohistochemistry of T Cell Lymphomas, M. Phil. Thesis. Vol 12. Bangladesh: University of Dhaka; 1991. p. :34-39.
- [Google Scholar]
- Angiogenesis extent and macrophage density increase simultaneously with pathological progression in B-cell non-Hodgkin's lymphomas. Br J Cancer. 1999;79(5-6):965-970.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution of fibronectin and laminin in normal and pathological lymphoid tissue. J Clin Pathol. 1985;38(08):849-854.
- [CrossRef] [PubMed] [Google Scholar]
- Angiogenesis spectrum in the stroma of B-cell non-Hodgkin's lymphomas. An immunohistochemical and ultrastructural study. Eur J Haematol. 1996;56(1-2):45-53.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular density (tumor angiogenesis) in non-Hodgkin's lymphomas and florid follicular hyperplasia: a morphometric study. Leuk Lymphoma. 2000;40(1-2):157-166.
- [CrossRef] [PubMed] [Google Scholar]
- A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin's lymphoma. Blood. 1997;90(08):3167-3172.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous elevation in the serum concentrations of the angiogenic growth factors VEGF and bFGF is an independent predictor of poor prognosis in non-Hodgkin lymphoma: a single-institution study of 200 patients. Blood. 2000;96(12):3712-3718.
- [CrossRef] [PubMed] [Google Scholar]
- Angiogenic growth factors and endostatin in non-Hodgkin's lymphoma. Br J Haematol. 1999;106(02):504-509.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of vascular endothelial growth factor in lymphomas and Castleman's disease. J Pathol. 1997;183(01):44-50.
- [CrossRef] [Google Scholar]
- Vascular endothelial growth factor expression in low and high grade B-cell non-Hodgkin's lymphomas. Turk Patoloji Derg. 2011;27(03):204-209.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of angiogenesis markers in the outcome of patients with diffuse large B cell lymphoma: a retrospective study of 97 patients. J Cancer Res Clin Oncol. 2008;134(03):381-387.
- [CrossRef] [PubMed] [Google Scholar]
- Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B-cell non-Hodgkin's lymphomas. Int J Cancer. 2000;85(02):171-175.
- [CrossRef] [Google Scholar]
- Prognostic significance of VEGF, VEGF receptors, and microvessel density in diffuse large B cell lymphoma treated with anthracycline-based chemotherapy. Lab Invest. 2008;88(01):38-47.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med. 2004;351(03):250-259.
- [CrossRef] [PubMed] [Google Scholar]
- VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol. 2007;128(06):966-973.
- [CrossRef] [PubMed] [Google Scholar]





