Translate this page into:
Human Herpesviruses as Copathogens of HIV Infection, Their Role in HIV Transmission, and Disease Progression
Address for correspondence: Prof. Sarman Singh, E-mail: sarman_singh@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Of eight human herpesviruses (HHVs), often, only herpes simplex virus types 1 (HSV-1) and 2 (HSV-2) find mention in medical literature as both of these viruses are commonly associated with genital lesions and oral ulcers, commonly known as cold sores. However, role of human herpesviruses as copathogens and in aggravation and in the transmission of other human diseases, especially the Acquired immunodeficiency syndrome (HIV/AIDS) has only very recently been recognized. Therefore, screening and treating subclinical HHV infections may offer slowing of HIV infection, disease progression, and its transmission. Beside HSV-1 and HSV-2, HHV-3 a causative agent of herpes zoster remained one of the first manifestations of HIV disease before the era of highly active antiretroviral therapy (HAART). HHV-5 also known as human Cytomegalovirus infection remains a significant risk factor for HIV-associated mortality and morbidity even in HAART era. It is proposed that Cytomegalovirus viremia could be a better predictor of HIV disease progression than CD4+ T-lymphocyte count. The role of HHV-4 or Epstein–Burr virus and HHV-6, HHV-7, and HHV-8 is still being investigated in HIV disease progression. This review provides insight into the current understanding about these 8 HHVs, their co-pathogenesis, and role in HIV/AIDS disease progression. The review also covers recent literature in favor and against administering anti-HHV treatment along with HAART for slower AIDS progression and interrupted sexual transmission.
Keywords
Acquired immunodeficiency syndrome
blood
Cytomegalovirus
herpesviruses
highly active antiretroviral therapy
human immunodeficiency virus
semen
transmission
INTRODUCTION
After almost 33 years of research on human immunodeficiency virus (HIV) epidemic in both developed and developing countries, it has become increasingly evident that both transmission of HIV[1] and its progression to acquired immunodeficiency syndrome (AIDS) are assisted by several biological factors, one of the most important factor is opportunistic infections (OIs).[2] These opportunistic infections are more frequent in developing countries.[34]
Another important factor is sexually transmitted infections (STIs). The STIs are associated with increased risk of HIV-1 transmission.[5] HIV-1-infected individuals with STIs tend to shed HIV-1 RNA 2–3 times more frequently in their genital secretions, as compared to HIV-infected individuals without an STI, making them more likely to transmit HIV.[6] Among viral STIs, only herpes simplex virus-2 (HSV-2) has been studied extensively for its role in HIV acquisition, transmission, and pathogenesis.[7] However, the role of human herpesviruses (HHVs) as asymptomatic viral STIs (other than HSV-2) has not been given due importance despite their high seroprevalence in HIV-infected individuals[8] and in the general population. In the early 1980s, severe clinical expressions of HHVs-8 (Kaposi's Sarcoma [KS]) infection were among the first recognized manifestations of AIDS.[9] Later, infections with various HHVs, such as HSV infection, KS, Burkitt's lymphoma, and Cytomegalovirus (CMV) retinitis, were included by the centers for disease control and prevention in the list of AIDS-defining conditions.[9] However, a comprehensive data on these HHVs and their role in HIV disease progression is lacking.
NATURAL HISTORY OF HUMAN IMMUNODEFICIENCY VIRUS INFECTION
In this paper we review the current literature from Indian and globally on this neglected or emerging aspect. The natural course of HIV infection is characterized by the unique interplay between the virus and several host factors as illustrated in Figure 1. Primary (or acute) HIV infection is associated with a precipitous drop in absolute CD4+ T cells count (blue line) and a rise in HIV viremia (red line). After the initial acute phase of infection which lasts between 6 and 9 weeks, HIV viremia drops to a lower level called the viral set-point. Establishment of a viral set point typically results in an increase in absolute CD4+ T cells counts, which subsequently declines over the course of infection. A drop of CD4+ T cells count below 200/mm3 is defined as clinical AIDS, which is associated with a rapid rise in viremia and decline in absolute CD4+ T cells counts.[1011]
![Natural course of human immunodeficiency virus infection and associated disease progression[10]](/content/164/2016/8/1/img/JLP-8-5-g001.png)
- Natural course of human immunodeficiency virus infection and associated disease progression[10]
Clinically, AIDS is defined by extreme immunodeficiency leading to increased incidences of malignancies and OIs.[12] Although still unclear, it is believed that the myriad of OIs associated with advanced HIV disease may be responsible for the constitutional symptoms reported by almost half of all individuals[1213] [Figure 2]. Though the clinical course of HIV disease and pattern of OIs varies from patient to patient and country to country.[41314] For instance, the clinical profile of AIDS individuals in developing countries like India includes a wide range of conditions such as tuberculosis, coccidian diarrhoea, cryptococcal meningitis, papular pruritic eruptions, and CMV retinitis, among others.[3] While in developed countries, non-tuberculous mycobacteriosis and viral co-infections are more common.
![Opportunistic infections associated with advanced human immunodeficiency virus disease [adapted from http://www.microbiologybook.org/lecture/images/natural-history.gif]](/content/164/2016/8/1/img/JLP-8-5-g002.png)
- Opportunistic infections associated with advanced human immunodeficiency virus disease [adapted from http://www.microbiologybook.org/lecture/images/natural-history.gif]
Due to a scarcity of data from India, the current belief is that the HHV prevalence is quite low in this subcontinent, which is in contrast to the global epidemiology. The present review will increase our understanding of the epidemiology of HHVs and the manifestations associated in this “Treat All Era.”
ROLE OF HHVS IN HIV TRANSMISSION AND DISEASE PROGRESSION
In a healthy human body, microbes maintain an effective parity with the host, when a new microbe pervades, but for some the host fails to readjust this parity, and these microbes become pathogens (copathogens).[15] HIV-induced disruption of the equilibrium between a human host and its virome leads to two grave consequences: (1) Reactivation of ubiquitous viruses (copathogens), which start to replicate to higher levels, for example, HHVs and (2) infection by new viruses (coinfections).[16] In this imbalanced system, the reactivation of copathogens leads to different pathological conditions, including some “AIDS-defining” ones. The data accumulated over recent years on the mechanisms of the interaction between coinfecting microbes, in particular the HIV, suggest that non-HIV viruses may determine HIV transmission, its pathogenesis, and evolution.[15] In addition, there are several viral and host factors which determine variability in transmission to the partner and disease progression in the HIV-infected individuals. Hence, the spread of HIV is assisted by several biological factors, one of the most important factors is STIs, both symptomatic and asymptomatic.[6] STIs can increase the risk of acquisition and transmission of HIV,[1718] via a number of mechanisms, including breach of mechanical barriers to infection, increased inflammation and higher levels of HIV cellular targets,[19] and increased HIV viral load in genital tract.[20] On the infectiousness side, STIs might evoke a more infectious HIV variant[21] and can increase HIV concentrations in genital lesions, semen, or both.[22] Acute HIV infection has been found to be more frequent in individuals with active STIs, and cotransmission is a common phenomenon.[23]
The interactions between HHVs and HIV-1 are increasingly being recognized;[242526] As it will be discussed in the next section, there are many ways in which these viruses could potentially interact with HIV. When viewed from the perspective of viral taxonomy, there is no obvious connection between members of the Retroviridae and Herpesviridae. To study each of the eight known HHVs in relation to the clinical manifestation, their role in HIV pathogenesis, heterosexual transmission and how they subvert the immune system following HIV infection would be an enormous undertaking. Therefore, we have tried to crystallize the data related to HHVs in the context of HIV infection, their role in fuelling the HIV epidemic, and the direction of research we can expect to see soon.
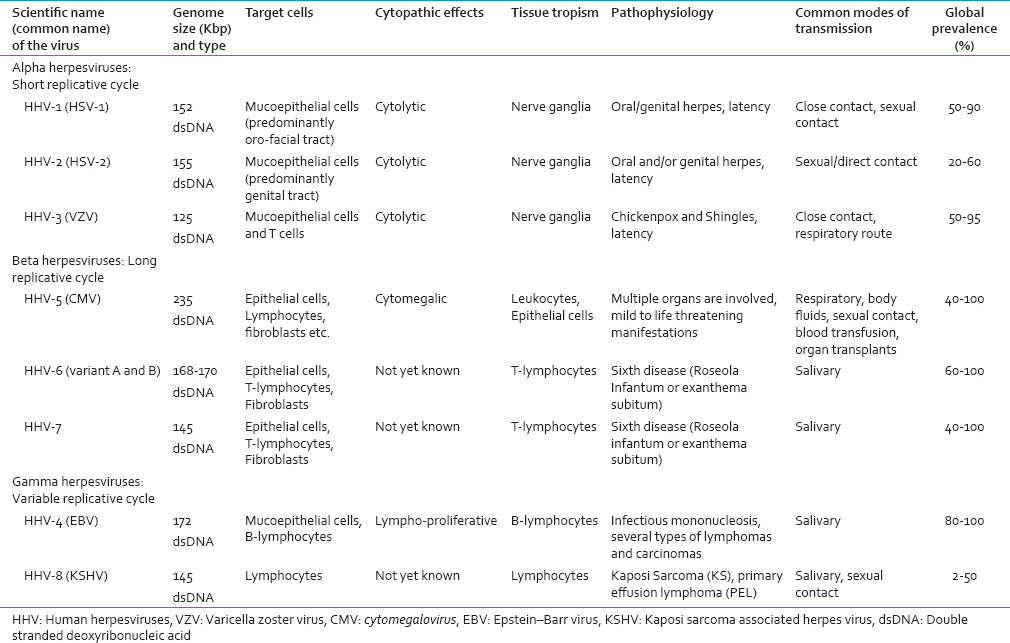
HUMAN HERPESVIRUSES
Herpesviridae is a large family of DNA viruses that cause diseases in animals, including humans.[27] In 2009, the family Herpesviridae was elevated to the order Herpesvirales.[28] The order currently has 3 families, 3 subfamilies (plus 1 unassigned), 17 genera, 90 species, and 48 unassigned viruses.[29] Based on their biological properties such as growth characteristics and tissue tropism, herpesviruses can be further divided into three subfamilies;[29] alpha-(α), beta-(β) and gamma-(γ) herpesvirinae as illustrated in Table 1. Among the eight HHVs, the alpha subfamily includes neurotropic viruses and contains the HSV-1 and 2 and varicella zoster virus (VZV).[30] The members of the gamma subfamily are lymphotropic viruses and include Epstein–Barr virus (EBV) and HHV-8. The viruses of the beta subfamily appear to be able to establish infections in many different types of cells and tissues and include CMV, and HHV-6 and HHV-7.[30] A characteristic feature common to all herpesviruses is that after primary infection often during childhood, a latent phase is developed that persists lifelong in the infected host.[30] The host reservoir for latent viruses differs depending on the subfamily of a herpesvirus. Occasionally, the latent virus is reactivated resulting in recurrent symptomatic or nonsymptomatic productive infections.[31] One explanation behind this coexistence between the herpesviruses and their host is a profound ability of these viruses to modulate the host immune response, interfere, and interact with different immune effectors, thereby promoting viral persistence in its host.[15]
POSSIBLE ROLE OF HUMAN HERPESVIRUSES IN HIV PATHOGENESIS AND TRANSMISSION
Preceding HIV infection, the dynamic balance between the human virome and the immunocompetent host is determined by the capability of the immune system to induce effective responses and the ability of herpesviruses to escape them.[32] Thus, the immunologic impact of viral coinfections has the amplitude to increase viral replication, viral genotypic heterogeneity, and CD4+ T-lymphocyte loss, leading to expedited atrophy in immune function, reduced survival, and added HIV-1 transmission risk.[33] Over the last 33 years, epidemiologic and molecular studies have indicated a strong and synergistic relationship between the dual epidemics of HSV-2 and HIV-1 infection.[34] The synergy between these two STIs goes beyond similar risk factors for acquisition; they interact both in their epidemiologic niche and at a pathogenesis level driving HIV replication.[35]
The molecular mechanisms by which various HHVs escape the immune responses have been described in detail elsewhere.[16] Although the immune system imbalance caused by HIV fails to build an adequate immune response, the system is not at a standstill. It responds to viral reactivations and new infections with poorly coordinated but powerful elements of the previously effective defense.[15] Reactivated HHVs activate the CD4+ T cells, which become new targets for HIV.[36] Also HIV-1 infection promotes HSV-2 genital shedding, which, in turn, generates an influx of activated T cells that serve as new cell targets for HIV replication.[37] Likewise, in CMV/HIV-1-coinfected individuals, the increase in the fraction of specific CMV-activated T cells and the higher rate of T cell proliferation in response to CMV are associated with accelerated CD4+ T cell loss and progression to AIDS.[38] Thus, apparently, in HIV-1-infected individuals, unceasing efforts of the host to overturn formerly controlled HHVs, lead to chronic cell immune activation.[39] In HIV-1-infected individuals, the chemokine/cytokine spectrum, which is already modulated in response to HIV, is changed further upon reactivation of the HHVs in a failed attempt to control these viruses. For example, HHV-6 specifically up-regulates CCL5.[40] Female genital tract secretions may provide innate protection against HIV infection through the activity of antimicrobial proteins and peptides whereas seminal plasma proteins may promote HIV infection by interfering with female host defenses and enhancing the infectivity of viral particles.[41] In the genital tract, HSV-2 interferes with the secretion of several cytokines,[42] and semen deposition results in modulated immunity and an inflammatory response of the genital mucosa. These semen-induced alterations in the female reproductive tract, thus, have implications for the HIV-1 sexual transmission.[43]
The effect of an imbalanced chemokine/cytokine system on HIV disease is difficult to study because of the complexity and redundancy of this system and also because of the additional effects of virokines and viroceptors. For example, HHV-6 encodes both virokine UL83A, which binds to CCR5, and viroceptor U51, which may act as ''chemokine sink'' for CCL5,[44] whereas CMV US28 viroceptor offers an additional coreceptor for HIV-1.[45] Similarly, viral proteins may shortcut the host immune response and directly affect HIV-1 transcription by transactivating HIV-1 long terminal repeat (LTR) or by interfering with Tat functions. Viral proteins capable of LTR transactivation have been identified in HSV,[46] CMV,[47] HHV-6,[48] and HHV-8[49] genomes [Table 2].
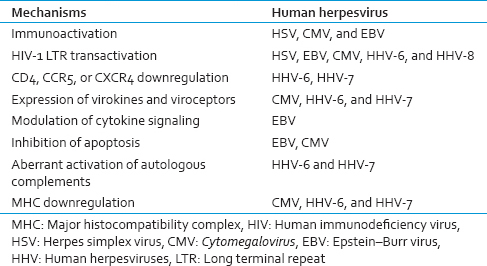
On the other hand, HIV-1-encoded proteins that also affect coinfecting HHVs. For example, HHV-6 suppresses HIV by upregulating CCR5 blocking chemokine.[40] In HIV disease, the reactivation of HHVs significantly contributes to the immune dysfunction of the HIV-1-coinfected host.[24] The effect of an imbalanced chemokine/cytokine system on HIV disease is difficult to study because of the complexity and redundancy of this system and also additional effects of virokines and viroceptors. Thus, the immunodeficiency primarily caused by HIV results in reactivation of viruses and new infections, further impairing the immune system and thus leading to one of the several vicious cycles that fuel HIV and coinfecting HHVs leading to disease progression [Figure 3].
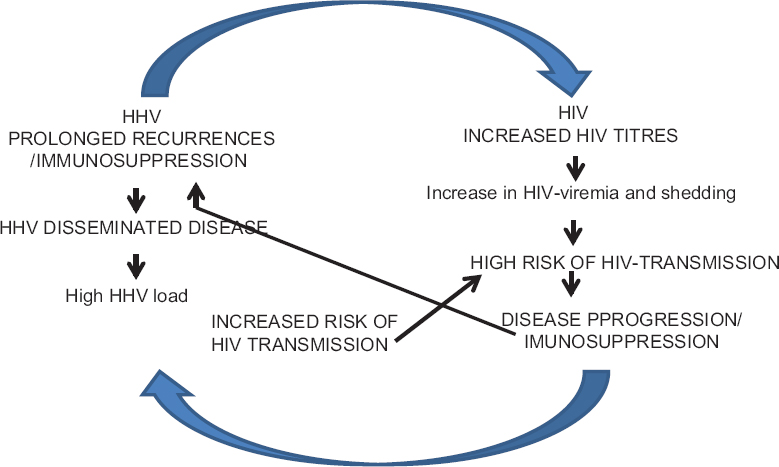
- The interaction between human herpesviruses and human immunodeficiency virus
EPIDEMIOLOGY AND CLINICAL MANIFESTATIONS OF HUMAN HERPESVIRUSES
HHVs are among the most ubiquitous of human infections. During the past several years, there have been rapid advances in our understanding of HHVs infection. Distinct variations in worldwide distribution of herpesviruses have been observed in HIV-infected and HIV-uninfected population as discussed below.
THE HERPES SIMPLEX VIRUS
Both HSV-1 and HSV-2 infect millions of people worldwide. Globally, the seroprevalence of HSV-1 and HSV-2 in the general population is around 70–100% and 6–50%, respectively.[50] HSV-1 prevalence is almost uniform around the globe and is more prevalent than HSV-2 infection in all general population studied.[51] Among adolescents and adults >15 years of age, HSV-1 seroprevalence is very high (more than 90%) in Central Africa, Eritrea, and Uganda.[52] In Asia, the prevalence of HSV-1 among sexually transmitted disease (STD) clinic attendees in Osaka, Japan, has been found to be 73%.[53] In India, no difference in HSV-1 prevalence among high-risk population versus general population has been reported, being 91.5% and 91.8%, respectively.[54]
HSV-2 seroprevalence is higher in the USA than in Europe;[55] Africa has the highest prevalence and Asia has the lowest prevalence rates.[5657] For the general population, it is 16.2% in the USA,[58] 12% in Australia,[59] 24.4% in Nigeria,[56] and 13.2% in China.[60] Similar prevalence rates have been observed in India, the prevalence of HSV-2 in the general adult population ranges from 5.2% to 14.5%.[6162] It has been observed that HSV-2 seroprevalence increases steadily after sexual debut and with age.[58] In Sub-Saharan Africa, half of the general population is HSV-2 seropositive by the age of 25 years.[57]
Nevertheless, prevalence rates ranging from 52% to 95% for HSV-2 have been reported among HIV-infected individuals worldwide.[6364] In Sub-Saharan Africa, more than 85% of HIV-1-infected individuals are HSV-2 seropositive.[55] In the developed countries, the HSV-2 prevalence ranging from 65% to 80% has been reported in HIV-1-infected men.[65] In India, the seroprevalence of HSV-2 is reported to be as high as 83% among the STD clinic attendees.[66] We have found prevalence of HSV-1 and HSV-2 in HIV infected vs HIV uninfected Indian men to be 78.6% vs 34% and 70.8% vs 5%, respectively.
In terms of clinical manifestation, genital herpes (GH) may be caused by either HSV-1 or HSV-2 but, globally, a majority of the cases are caused by HSV-2, infection is common in both industrialized and developing world.[67] However, with the rise in socioeconomic conditions and change in sexual practices, changes in HSV-1 epidemiology have been observed, showing acquisition in later age.[68] The studies have confirmed that HSV-1 accounts for 42% and 62% of confirmed first episode of GH due to HSV-1 and also the proportion of newly diagnosed GH in young adults due to HSV-1 has risen dramatically.[69] In developing countries, the proportion of GH caused by HSV-1 is unknown.
HSV leads to severe morbidity and mortality, both in the setting of advanced HIV disease and in immunocompetent hosts. Apart from GH in high-risk individuals, more serious and systemic manifestations of HSV infection include esophagitis, meningoencephalitis, hepatitis, pneumonitis, retinal necrosis, and disseminated infection, all of which are relatively rare, even among those with advanced disease.[9] Atypical and disseminated HSV infection occur relatively more often in HIV-infected individuals.[70]
VARICELLA ZOSTER VIRUS
VZV causes two distinct diseases, varicella (chickenpox) and herpes zoster (HZ) or shingles a very painful and debilitating condition.[71] In both temperate and tropical climates, the peak disease incidence is most commonly reported in the colder, and drier months during winter or spring. Periodicity with interepidemic cycles of 2–5 years is described from many countries.[71] In temperate climates, the highest incidence of varicella occurs among children aged <10 years, leading to seroprevalence of more than 90% before adolescence.[72] However, in countries with tropical climates, a majority of the studies have described later acquisition of varicella in childhood with a higher proportion of cases and higher susceptibility among adults.[72] In Thailand, the overall prevalence rate of 52.8% has been observed in the general population aged <30 years, ranging from 15.5% in children <5 years of age to 75.9% in young adults aged 20–29 years.[73] A study from India has reported similar rates (69.2%) at 20–29 years of age to as high as 96.1% at 30–39 years of age.[74] A significant proportion of adolescents and adults is susceptible to varicella in India as observed in other tropical countries. In our cohort the seroprevalence was not found to be different between HIV infected and HIV uninfected men; 78.6% and 76.6%, respectively. Although the statistical evidence from tropical countries is sparse, belief is that adulthood varicella is more severe than childhood varicella in tropical countries.[75]
HZ develops primarily in healthy adults after the age of 50 and in HIV-infected persons of all ages and at all stages.[7275] Studies using varied methods and conducted in different population around the world have revealed a median zoster incidence of 4–4.5/1000 person-years.[76] In India, the incidence of HZ is observed mainly in the fourth and third decades of life, with the maximum incidence in the age group of 31–40 years.[77] It has been observed that in India, more males are affected than females,[77] in contrast, to the Western studies,[78] where both males and female are equally affected. Trauma, stress, and outdoor activities may be the predisposing factor for the male preponderance in Indian setup.
The HIV epidemic may also affect the epidemiology of varicella.[75] In countries with high HIV prevalence such as Sub-Saharan Africa and India, varicella may cause more severe morbidity and mortality; however, there are few population-based data examining these issues. The risk for zoster and its recurrence is elevated in persons infected with HIV.[79] The Asian with high HIV prevalence may experience greater morbidity and mortality rates due to VZV, especially due to its acquisition at a later age.[7580] A recent study on 200 HIV-positive individuals from eastern India reported VZV incidence rate of 32.5% among adults.[81]
In AIDS individuals, VZV retinitis results in blindness in 75–85% of involved eyes.[82] VZV can also lead to a variety of atypical cutaneous lesions in HIV-infected individuals with low CD4+ T-lymphocyte counts.[80] These lesions may be chronic, persisting for months or years, and are sometimes associated with acyclovir-resistant strains of VZV.[83]
EPSTEIN–BARR VIRUS
EBV is an ancient virus and has probably coevolved with its different hosts over the last 90–100 million years.[84] With the ability to establish lifelong latency and intermittent reactivation after primary infection and with limited clinical symptoms in the majority of infected individuals. Large-scale seroepidemiological surveys in the United States[85] and Europe[86] have showed that over 50% of the adolescents are seropositive to EBV. A high EBV seropositivity rate ranging from 60% to 93% has been reported from South Asia.[87] In developed countries, a bimodal infection rate, with peaks in children below 5 years and again after 10 years of age, has been described.[85] Poor socioeconomic conditions have been associated with early primary EBV infection whereas late primary EBV infection is seen in population of high socioeconomic status.[26] Low income and crowded family conditions have also been found to increase the likelihood of being EBV seropositive in children from Asian locales.[88] In countries with high HIV burden such as South Africa and Chile, the prevalence of EBV in HIV-infected individuals ranges from 90% to 100%.[8990] In a recent cross-sectional survey conducted to determine the seroprevalence of various viral coinfections among HIV-infected individuals, EBV prevalence of 96.6% was observed in China.[91] We also found a very high seropositivity of 98% and 92.5% in HIV positive and HIV negative males, respectively. No well-defined studies from India have been carried out in HIV-infected individual to assess the comorbidities associated with EBV. The virus is associated with various types of malignancies such as Burkitt's lymphoma, Hodgkin's lymphoma, Nasopharyngeal carcinoma etc.
The association between EBV and HIV markers reflects a significant pathogenic interaction between the two viruses.[92] With the availability of highly active antiretroviral therapy (HAART) and treatment and prevention of OIs, both an increase in life expectancy of HIV-infected individuals and an increase in HIV-related malignancies are expected.[93] Although lymphomas have been reported in individuals with acquired immunodeficiency syndrome, they have rarely been reported from the Indian subcontinent.[94] Given huge population of people living with HIV and high incidence of EBV in these individuals,[81] closer attention should be paid; the wide spectrum of changes in AIDS-related lymphadenopathy requires recognition.
CYTOMEGALOVIRUS
The human cytomegalovirus (HCMV) is the most ubiquitous agent of all HHVs. The overall prevalence and age of initial CMV infection vary significantly in different regions of the world. General trends have tended to show seroprevalence rates ranging as low as 40% to as high as 100%, with lower rates in Europe,[95] North America,[96] and Australia[97] and higher rates in Africa[26] and Asia.[98] A study on voluntary blood donors from Delhi, India, reported the prevalence of 95% in individuals over 18 years of age,[99] and similar rates have been reported from developing countries indicate rates approaching 100% by the age of 11 years.[2695] As CMV infection is highly prevalent and HIV and CMV share several modes of transmission, it is not surprising that CMV seroprevalence is extremely high in HIV-infected population.[8189] CMV infection is prevalent not only in HIV-infected population, but also in those populations that are at risk for HIV infection; approximately 75% of injection drug users and more than 90% of homosexual men who are infected with HIV have detectable IgG antibodies to CMV.[14100] In addition, high CMV incident rates, in longstanding CMV-seropositive homosexual men,[101] suggest that this group is frequently reexposed to differing exogenous strains of CMV.[102]
Reactivation of CMV infection in the AIDS individuals usually presents as retinitis or gastrointestinal involvement, which further may lead to CMV end-organ disease and death as a consequence of the impaired immunity.[103104] A study showed that CMV, rather than Helicobacter pylori, may be the main causative pathogen of peptic ulcers in HIV-infected individuals.[105]
In the intervening years, especially with the advent of effective HAART, interest in the CMV cofactor hypothesis for HIV progression has waned. The timely study conducted on HIV-infected adults with CMV seropositivity rate of more than 90% by Fowotade et al. revisits the issue of active CMV infection in HIV-infected individuals in the HAART era.[106] Although the introduction of HAART has reduced the proportion of individuals at risk for reactivation of CMV disease. Even in a well-defined, HIV-infected population, it is associated with sustained low CD4+ T cell counts at risk for CMV disease. Recently, several studies have shown that neurological and ocular complications are rare in the treatment period, but they continue to occur for several reasons: (1) individuals fail to use HAART, either because they are not in care or are nonadherent, (2) antiretroviral resistance, and (3) late HIV diagnosis (late presenters).[82107]
HUMAN HERPESVIRUS-6
HHV-6 first isolated from individuals with lymphoproliferative disorders in 1986 is a human pathogen of emerging clinical significance.[108] HHV-6 is widespread throughout the world, with geographic differences in HHV-6 prevalence varying between 65% and 100%[109] in children <1 year.[110] The prevalence was 66.0% in individuals aged 11–40 years; however, no HHV-6 antibodies were detected in subjects >60 years, suggesting decline in HHV-6 prevalence with advancing age.[110] HHV-6 prevalence in healthy population from Northern China is reported to be 69.1%.[111] Compared to Malaysian study, here, no significant difference in prevalence was observed in children and adults of various age groups, suggesting that HHV-6 infection is ubiquitous in China. Exceptions if confirmed are represented by Morocco where seroprevalence is much lower (20%).[112] No significant difference in prevalence among ethnic groups living in the same geographical location or between sexes has been reported so far.[113] We for the first time found very high seroprevalence of HHV-6. The prevalence was high in both HIV positive (98.7%) and HIV negative (95%) males.
HHV-6 has two variant groups (A and B)[114] while HHV-6B is present in almost 100% of the world's population, HHV-6A appears to be less frequent in Asia, North America, and Europe.[114] In addition, there are virulence distinctions between variants HHV-6A and B, with evidence for increased severity and neurotropism for HHV-6A.[115] Primary infant infections in Europe, the USA, and Japan are predominantly HHV-6B (97–100%) while, in Africa, the reverse has been observed, as high as 86–100% of healthy infants acquire HHV-6A as their primary HHV-6 infection.[116]
The epidemiology and disease associations of HHV-6A are less well defined. HHV-6 infection/reactivation in AIDS individuals results in an increase in HHV-6 load both in lymph nodes and generalized, in viremia, disseminated infection in many organs, active CNS infection, pneumonitis, and retinitis and even a cause of death.[109] HHV-6-related bilateral posterior uveitis as well as optic neuritis has been reported in HIV-infected individuals both with or without clinical manifestations of AIDS.[117] These findings lead to the proposal that HHV-6 acts as a cofactor in the progression of AIDS and the switch of HIV from the latent to the replicative state.[118] Despite the bulk of data hitherto accumulated, conclusive evidence of the role played in the progression of HIV-1 disease is still lacking.
HUMAN HERPESVIRUS-7
HHV-7 was recently discovered in the year 1990.[119] It is a beta herpesvirus similar to but distinct from HHV-6.[120] It is closely related to herpesvirus 6B and replicates in human cells only after binding CD4+ T cell receptor. It establishes lifelong latency in infected cells, and its frequent reactivations result in asymptomatic virus shedding through saliva. But very little is known about its prevalence, biology, immunology, and molecular biology aspect.[121] The peak age of initial infection for HHV-7 is slightly later than that for HHV-6; the most common age range for infection is 18 months to 3 years of age.[122] By the age of 5 years, >90% of the US population demonstrate evidence of HHV-7 infection.[121] In a single study, healthy blood donors from nine countries in five continents were studied for HHV-7 prevalence. The prevalence for HHV-7 was high (75–98%) in practically all countries except for Northern Japan (44%).[123] The study also showed that there are regions of low, intermediate, and high mean antibody titers against HHV-7 such as lowest for Belgium, Israel, Japan, the USA, and Australia, high for Mexico and Cologne/Germany, and highest for South Africa, for which geographic characteristics may be responsible.[123] Hence, HHV-7 similar to HHV-6 is a widespread HHV with elevated antibody titers in the healthy human population essentially everywhere.
So far, from Asia, there are only two seroprevalence studies, one study from Thailand conducted during 1990–1993, in which 333 serum samples from umbilical cord blood and venous blood of healthy persons were investigated.[124] The study showed that by the age of 5 years, >80% of the children are positive, however, the seroprevalence starts declining with age, reaching 67% in individuals over 30 years of age.[124] Another study in healthy children and adults in Japan showed that seroprevalence was highest (60%) at 11–13 years of age and was maintained until the end of the third decade, then decreased thereafter.[125] We found that seroprevalence of HHV-7 was. similar in both HIV positive (86%) and HIV negative (79.1%) males.
Besides the HHV-7 etiologic role in a few cases of exanthema subitum[30114] and chronic fatigue syndrome,[126] primary HHV-7 infection can cause serious neurologic disease.[127] HHV-6B infection is a common cause of febrile status epilepticus (FSE), but HHV-7 infection is also associated with FSE.[128] Together, they account for one-third of FSE, a condition associated with an increased risk of both hippocampal injury and subsequent temporal lobe epilepsy.[128] Evidence suggests HHV-7 is associated with herpetic (non-CMV) retinal infections,[129] oral ulcers and other dermatological manifestations in AIDS patients.[130]
HUMAN HERPESVIRUS-8 OR KAPOSI SARCOMA-ASSOCIATED HERPESVIRUS
HHV-8 is considered to be the primary etiological agent of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease.[131] There exists a wide geographic variation in the prevalence of HHV-8 whether it is a region of high endemicity or a nonendemic region.[132] For example, in Uganda, where KS is endemic, HHV-8 seroprevalence rates of 50% have been reported for the general population whereas seroprevalence rates of <5% or lower have been reported from the USA and Northern Europe and Asia.[132] In endemic countries as in the Mediterranean basin (Italy, Greece) and Africa (East and Central Africa), HHV-8 seroprevalence increases with age and often reaches adult rates before the end of puberty.[132] HHV-8 infection is associated with high-risk sexual practices.[133] In nonendemic countries such as the United States and Western Europe, infection is highly prevalent ranging from 20% to 60% among high-risk individuals.[132] Historically, KS has been considered to occur in individuals infected with HIV, who have low CD4+ T cell counts and high viral loads.[134] However, emerging data show that KS also occurs in HIV-positive individuals with higher CD4+ T cell counts and undetectable viral loads. The seroprevalence of HHV-8 in 165 Indian adult males was estimated, using ELISA and IFA methods. Of these, 43 (26.06%) males were positive by ELISA while 26 males (15.8%) were also positive by IFA. Seroprevalence decreased with increasing age (P<0.05). Factors independently associated with HHV-8 infection were younger age group and alcohol consumption. These findings suggest that even in heterosexual population, HHV-8 can transmit frequently. Most important observation of the study was comparable rates of HHV-8 infection in heterosexual men and MSM.[135]
THERAPEUTIC MANAGEMENT OF HUMAN HERPESVIRUSES
Before considering treatment of HHV infections, it is important to establish the specific diagnosis. Various types of clinical samples and laboratory technologies are used for this, as summarized in the Table 3. The antiviral agents act by impeding entry of viruses into host cells; interfering with viral assembly, release, or deaggregation; inhibiting transcription or replication of the viral genome; or interrupting viral protein synthesis. Antiviral agents can be used to treat disease (a therapeutic strategy), prevent infection (a prophylactic strategy), or prevent disease (a preemptive strategy). In the preantiviral era, the widely held belief was that any therapeutically meaningful interference with viral replication would destroy the host cell upon which viral replication was dependent.[136] A growing understanding of host cell–virus interactions and viral replication has led to the development of safe and effective antivirals. There are a number of antiviral medications, with activity against HHVs. With the exception of foscarnet and cidofovir, all are nucleoside analogs.
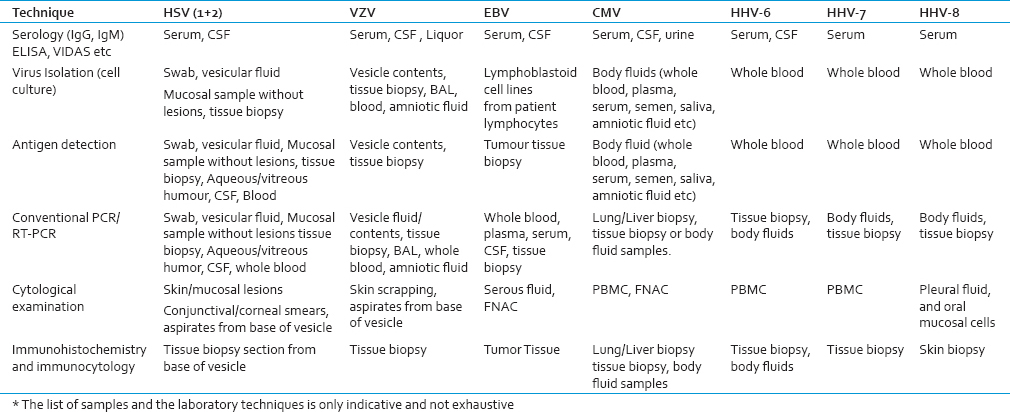
Treating herpesvirus coinfections has long been pursued as a potential strategy to delay HIV disease progression. Before the introduction of protease inhibitors in the mid-1990s, several small trials established a mortality benefit to high-dose acyclovir when used in combination with 1 or 2 antiretroviral drugs.[137] A recent meta-analysis study on seventeen trials all double blinded suggested favorable effects of acyclovir on plasma HIV-1 viral load among persons coinfected with HSV-2.[138] HSV-2 suppressive therapy could be an affordable strategy for reducing HIV-1 disease progression and retaining individuals in care before ART initiation.[139] Acyclovir has proved to be beneficial for GH/HIV-1 coinfected individuals by slowing down the rate of CD4+ T cell decline and, delaying of HIV disease progression.[140] A study suggested that acyclovir exhibits a direct antiviral effect on HIV replication as well.[141] Acyclovir may prove a useful lead for development of new HIV treatments but, the selection of resistant mutants raises a cautionary note to the use of acyclovir mono-therapy in individuals coinfected with HSV and HIV.[142] However, it is not fully known whether this is direct or indirect effect. This has led many to hypothesize that the beneficial effect of acyclovir on HIV progression is mediated by a reduction in HSV-2–induced immune activation.[140] A recent study on pooled analysis of episodic therapy trials showed acyclovir has little impact on the healing of GH; however, the impact was more substantial in HIV-1 seropositive individuals, with high CD4+ T cell counts.[143]
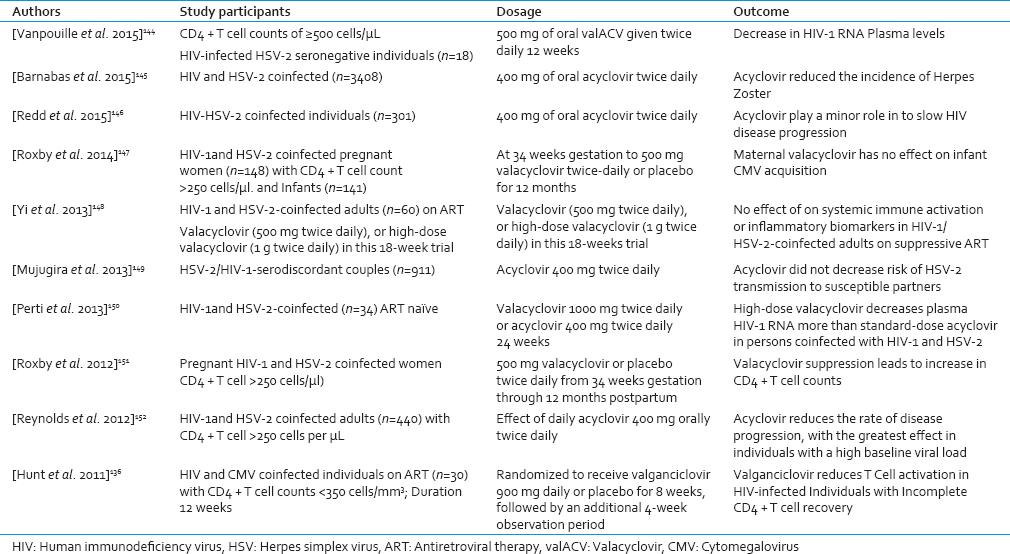
Results of a recent meta-analysis study showed a range of favorable effects of ACV or its prodrug valacyclovir (valACV) on plasma HIV-1 viral load among HIV-HSV-2 coinfected individuals.[138144] [Table 4]. However, the studies of whether antiviral therapy in combination with ART will prevent HHV-associated disease are needed.
CONCLUSIONS
The clinical management of HHVs in individuals with HIV infection has lagged seriously behind the large body of medical literature on the importance of the interaction between these pathogens. However, the recent data shows that screening and treating subclinical HHV infection may offer benefits to individuals with HIV infection. HSV is capable of causing severe morbidity and mortality, both in the advanced HIV disease as well as in immunocompetent hosts. HSV suppressive treatment can reduce HIV-1 infectiousness and delay disease progression in HIV-1-infected individuals. HZ remains the common complication of HIV disease as it used to be before the advent of HAART. The incorporation of the VZV vaccine into the routine care of HIV-infected individuals has been slow, due to fears surrounding the use of a live vaccine in this population. For this reason the CMV viremia remains a significant risk factor for progression of HIV disease and death even after HAART era. CMV DNA viremia is proposed to be used as a better disease predictor than CD4+ T-lymphocyte count in HIV-infected subjects. Given that, over 90% of HIV-infected individuals are seropositive for CMV infection, and increasing numbers of individuals are exhausting HAART options leading to decrease in CD4+ T cell counts, the ongoing threat of CMV disease must not be overlooked. Finally, with EBV, HHV-6, HHV-7, and HHV-8, the infections that are still overlooked, need to be studied specially for the effect of therapy on reducing the risk of certain malignancies. Many questions about the interaction between these organisms remain to be clarified, and larger studies with defined clinical endpoints are needed to understand the role of various HHVs in transmission and HIV disease progression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: How much really is known? Sex Transm Dis. 2001;28:579-97.
- [Google Scholar]
- Balancing the need to rapidly scale-up and improve clinical outcomes in antiretroviral programmes in developing countries: Lessons from an Indian programmatic cohort study. Trans R Soc Trop Med Hyg. 2014;108:599-600.
- [Google Scholar]
- The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sex Transm Dis. 2008;35:946-59.
- [Google Scholar]
- The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33-42.
- [Google Scholar]
- Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study) J Acquir Immune Defic Syndr. 2011;57:238-44.
- [Google Scholar]
- The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435-45.
- [Google Scholar]
- Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: Recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1-166.
- [Google Scholar]
- Past, present and future: 30 years of HIV research. Nat Rev Microbiol. 2013;11:877-83.
- [Google Scholar]
- AIDS-related and non-AIDS-related mortality in the Asia-Pacific region in the era of combination antiretroviral treatment. AIDS. 2009;23:2323-36.
- [Google Scholar]
- AIDS related opportunistic infections, going but not gone. Arch Pharm Res. 2002;25:215-28.
- [Google Scholar]
- Coinfecting viruses as determinants of HIV disease. Curr HIV/AIDS Rep. 2009;6:5-12.
- [Google Scholar]
- Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat Med. 2006;12:289-95.
- [Google Scholar]
- Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: A probabilistic empiric model. AIDS. 2001;15:621-7.
- [Google Scholar]
- Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol. 2011;65:308-16.
- [Google Scholar]
- Relationship between HIV-RNA load in blood and semen in antiretroviral-naïve and experienced men and effect of asymptomatic sexually transmissible infections. Curr HIV Res. 2008;6:138-42.
- [Google Scholar]
- Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol. 2000;74:8946-52.
- [Google Scholar]
- The genital tract immune milieu: An important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32-40.
- [Google Scholar]
- Amplified transmission of HIV-1: Comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723-30.
- [Google Scholar]
- Semen of HIV-1-infected individuals: Local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97-105.
- [Google Scholar]
- Italian Seroconversion Study. Effect of multiple herpesvirus infections on the progression of HIV disease in a cohort of HIV seroconverters. J Med Virol. 2003;69:182-7.
- [Google Scholar]
- Seroprevalence of HHV-8, CMV, and EBV among the general population in Ghana, West Africa. BMC Infect Dis. 2008;8:111.
- [Google Scholar]
- Herpesviridae. In: Fields BN, Knipe DM, Howley PM, eds. Fields Virology. Philadelphia, Pa.: Lippincott-Raven; 1996. p. :2221-30.
- [Google Scholar]
- Overview of classification. 2007. Human 1, Herpesviruses. Ch. 1.. Cambridge: Cambridge University Press; :1-8. Available at http://www.ncbi.nlm.nih.gov/books/NBK47375/?report=reader
- [Google Scholar]
- Field Virology Vol Vol. 2. (5th ed). New York: Lippencott-Raven; 2007. p. :2479-500.
- War and peace between microbes: HIV-1 interactions with coinfecting viruses. Cell Host Microbe. 2009;6:403-8.
- [Google Scholar]
- Developments in STD/HIV interactions: The intertwining epidemics of HIV and HSV-2. Infect Dis Clin North Am. 2005;19:415-25.
- [Google Scholar]
- Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589-98.
- [Google Scholar]
- Does HIV “piggyback” on CD4-like surface proteins of sperm, viruses, and bacteria. Implications for co-transmission, cellular tropism and the induction of autoimmunity in AIDS? J Theor Biol. 1993;160:249-64.
- [Google Scholar]
- HIV-1 induced activation of CD4+T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699-704.
- [Google Scholar]
- The relationship between T-cell levels and CMV infection in asymptomatic HIV-1 antibody-positive homosexual men. J Acquir Immune Defic Syndr. 1993;6:407-13.
- [Google Scholar]
- HIV disease progression: Immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114-7.
- [Google Scholar]
- Suppression of CCR5- but not CXCR4-tropic HIV-1 in lymphoid tissue by human herpesvirus 6. Nat Med. 2001;7:1232-5.
- [Google Scholar]
- Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199-230.
- [Google Scholar]
- Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710-20.
- [Google Scholar]
- Role of semen in HIV-1 transmission: Inhibitor or facilitator? Am J Reprod Immunol. 2011;65:292-301.
- [Google Scholar]
- Viral exploitation and subversion of the immune system through chemokine mimicry. Nat Immunol. 2001;2:116-22.
- [Google Scholar]
- Identification of a chemokine receptor encoded by human Cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874-8.
- [Google Scholar]
- Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology. 1992;186:788-91.
- [Google Scholar]
- Cytomegalovirus and human herpesvirus-6 trans-activate the HIV-1 long terminal repeat via multiple response regions in human fetal astrocytes. J Neurovirol. 1998;4:495-511.
- [Google Scholar]
- Enhancement of TAT-induced transactivation of the HIV-1 LTR by two genomic fragments of HHV-6. J Med Virol. 1996;50:20-4.
- [Google Scholar]
- Human herpesvirus-8 (Kaposi's sarcoma-associated virus) ORF50 increases in vitro cell susceptibility to human immunodeficiency virus type 1 infection. J Gen Virol. 2003;84(Pt 5):1123-31.
- [Google Scholar]
- Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl. 1990;69:19-36.
- [Google Scholar]
- Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J Infect Dis. 2002;186(Suppl 1):S3-28.
- [Google Scholar]
- Seroprevalence of viral childhood infections in Eritrea. J Clin Virol. 2000;16:49-54.
- [Google Scholar]
- An epidemiologic study of herpes simplex virus type 1 and 2 infection in Japan based on type-specific serological assays. Epidemiol Infect. 1998;120:179-86.
- [Google Scholar]
- High seroprevalence of HSV-1 and HSV-2 in STD clinic attendees and non-high risk controls: A case control study at a referral hospital in south India. Indian J Dermatol Venereol Leprol. 2005;71:26-30.
- [Google Scholar]
- An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805-12, A.
- [Google Scholar]
- Seroprevalence of herpes simplex virus type-2 among patients attending the Sexually Transmitted Infections Clinic in Jos, Nigeria. J Infect Dev Ctries. 2010;4:572-5.
- [Google Scholar]
- Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A-35A.
- [Google Scholar]
- Seroprevalence of herpes simplex virus type 2 among persons aged 14-49 years-United States, 2005-2008. 2010. MMWR Morb Mortal Wkly Rep. 59:456-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20414188
- [Google Scholar]
- Prevalence of infection with herpes simplex virus types 1 and 2 in Australia: A nationwide population based survey. Sex Transm Infect. 2006;82:164-8.
- [Google Scholar]
- Herpes simplex virus infections among rural residents in eastern China. BMC Infect Dis. 2011;11:69.
- [Google Scholar]
- Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex Transm Infect. 2003;79:286-90.
- [Google Scholar]
- Population-based seroprevalence of HSV-2 and syphilis in Andhra Pradesh state of India. BMC Infect Dis. 2010;10:59.
- [Google Scholar]
- The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS. 2001;15(Suppl 4):S97-108.
- [Google Scholar]
- Proportion of new HIV infections attributable to herpes simplex 2 increases over time: Simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(Suppl 1):i17-24.
- [Google Scholar]
- Prevalence and correlates of herpes simplex virus type-2 infection among men who have sex with men, san francisco, 2008. Sex Transm Dis. 2011;38:617-21.
- [Google Scholar]
- Herpes simplex virus 2 infection in HIV-seropositive individuals in Tamil Nadu, India. 2014. Int J Med Sci Public Health. 4:404-7. Available from: http://www.scopemed.org/?mno=172880
- [Google Scholar]
- Herpes simplex virus type 1 as a cause of genital herpes: Impact on surveillance and prevention. J Infect Dis. 2000;181:1454-7.
- [Google Scholar]
- Herpes simplex virus type 1 remains the principal cause of initial anogenital herpes in Edinburgh, Scotland. Sex Transm Dis. 2004;31:322-4.
- [Google Scholar]
- Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797-800.
- [Google Scholar]
- Disseminated herpes simplex infection in a HIV+patient. G Ital Dermatol Venereol. 2009;144:205-9.
- [Google Scholar]
- VZV: immunobiology and host response. 2007. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Ch. 39. Cambridge: Cambridge University Press; :1-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21348065
- [Google Scholar]
- The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am. 1996;10:571-81.
- [Google Scholar]
- Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am J Trop Med Hyg. 2001;64:131-6.
- [Google Scholar]
- Age related seroprevalence of antibodies to varicella in India. Indian Pediatr. 2000;37:714-9.
- [Google Scholar]
- Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health. 1998;3:886-90.
- [Google Scholar]
- What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
- [Google Scholar]
- Herpes zoster: A clinical study in 205 patients. Indian J Dermatol. 2011;56:529-32.
- [Google Scholar]
- The varicella-zoster virus: Systemic and ocular features. J Am Acad Dermatol. 1984;11(2 Pt 1):165-91.
- [Google Scholar]
- The incidence of, risk factors for, and sequelae of herpes zoster among HIV patients in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2005;40:169-74.
- [Google Scholar]
- Subclinical reactivation of varicella zoster virus in all stages of HIV infection. J Neurol Sci. 2011;304:22-4.
- [Google Scholar]
- Incidence of multiple Herpesvirus infection in HIV seropositive patients, a big concern for Eastern Indian scenario. Virol J. 2010;7:147.
- [Google Scholar]
- Prevention and treatment of VZV infections in patients with HIV. Herpes. 2001;8:32-6.
- [Google Scholar]
- Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J Mol Biol. 1995;247:443-58.
- [Google Scholar]
- Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208:1286-93.
- [Google Scholar]
- Changes in seroprevalence to four herpesviruses over 30 years in Swedish children aged 9-12 years. J Clin Virol. 2006;37:118-23.
- [Google Scholar]
- A large-scale seroprevalence of Epstein-Barr virus in Taiwan. PLoS One. 2015;10:e0115836.
- [Google Scholar]
- Risk factors for Epstein-Barr virus infection in Thai infants. Southeast Asian J Trop Med Public Health. 2003;34:395-7.
- [Google Scholar]
- High seroprevalence of human herpesviruses in HIV-infected individuals attending primary healthcare facilities in rural South Africa. PLoS One. 2014;9:e99243.
- [Google Scholar]
- High seroprevalence of Cytomegalovirus, herpes simplex type 1 virus and Epstein Barr virus infection among human immunodeficiency virus-infected adults. Rev Med Chil. 2010;138:809-14.
- [Google Scholar]
- Multiple viral coinfections among HIV/AIDS patients in China. Biosci Trends. 2011;5:1-9.
- [Google Scholar]
- Epstein-Barr virus and HIV-AIDS-associated diseases. Biomed Pharmacother. 2001;55:348-52.
- [Google Scholar]
- Lymphoma in HIV patients: Varied presentations. Indian J Med Paediatr Oncol. 2010;31:39-42.
- [Google Scholar]
- Congenital Cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46(Suppl 4):S6-10.
- [Google Scholar]
- Seroprevalence of Cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143-51.
- [Google Scholar]
- National serosurvey of Cytomegalovirus in Australia. Clin Vaccine Immunol. 2006;13:1181-4.
- [Google Scholar]
- Review of Cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202-13.
- [Google Scholar]
- Seroprevalence of Cytomegalovirus among voluntary blood donors in Delhi, India. J Health Popul Nutr. 2002;20:348-51.
- [Google Scholar]
- Prevalence- and gender-specific immune response to opportunistic infections in HIV-infected patients in Lesotho. Sex Transm Dis. 2010;37:454-9.
- [Google Scholar]
- Increased prevalence of Cytomegalovirus antibodies in homosexual men with syphilis: Relation to sexual behaviour. Scand J Infect Dis. 1984;16:381-4.
- [Google Scholar]
- Multiple infections by Cytomegalovirus in patients with acquired immunodeficiency syndrome: Documentation by Southern blot hybridization. J Infect Dis. 1984;150:952-3.
- [Google Scholar]
- Burden of HIV-related Cytomegalovirus retinitis in resource-limited settings: A systematic review. Clin Infect Dis. 2013;57:1351-61.
- [Google Scholar]
- Low prevalence of Helicobacter pylori but high prevalence of Cytomegalovirus-associated peptic ulcer disease in AIDS patients: Comparative study of symptomatic subjects evaluated by endoscopy and CD4 counts. J Gastroenterol Hepatol. 2004;19:423-8.
- [Google Scholar]
- High seropositivity of IgG and IgM antibodies against Cytomegalovirus (CMV) among HIV-1 seropositive patients in Ilorin, Nigeria. Afr Health Sci. 2015;15:1-9.
- [Google Scholar]
- Neurologic Cytomegalovirus complications in patients with AIDS: Retrospective review of 13 cases and review of the literature. Rev Inst Med Trop Sao Paulo. 2010;52:305-10.
- [Google Scholar]
- Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986;234:596-601.
- [Google Scholar]
- Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-45.
- [Google Scholar]
- Seroepidemiology of human herpesvirus 6 in a population seen in the University Hospital, Kuala Lumpur, Malaysia. Southeast Asian J Trop Med Public Health. 1996;27:91-5.
- [Google Scholar]
- Seroprevalence of human herpesvirus-6 in healthy population in two provinces of north China. Chin Med Sci J. 1997;12:111-4.
- [Google Scholar]
- Seroepidemiology of human herpesvirus-6 in pregnant women from different parts of the world. J Med Virol. 1991;34:194-8.
- [Google Scholar]
- Molecular biology of human herpesviruses 6A and 6B. Infect Agents Dis. 1993;2:343-60.
- [Google Scholar]
- Predominant human herpesvirus 6 variant A infant infections in an HIV-1 endemic region of Sub-Saharan Africa. J Med Virol. 2009;81:779-89.
- [Google Scholar]
- Human herpesvirus 6A accelerates AIDS progression in macaques. Proc Natl Acad Sci U S A. 2007;104:5067-72.
- [Google Scholar]
- Isolation of a new herpesvirus from human CD4+T cells. Proc Natl Acad Sci U S A. 1990;87:748-52.
- [Google Scholar]
- Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human Cytomegalovirus. Proc Natl Acad Sci U S A. 1992;89:10552-6.
- [Google Scholar]
- Human herpesvirus 7: Antigenic properties and prevalence in children and adults. J Virol. 1991;65:6260-5.
- [Google Scholar]
- Comparison of seroprevalences of human herpesvirus-6 and -7 in healthy blood donors from nine countries. Vox Sang. 1998;75:193-7.
- [Google Scholar]
- Seroprevalence of human herpesvirus 6 and 7 infections in the Thai population. Asian Pac J Allergy Immunol. 1995;13:151-7.
- [Google Scholar]
- Seroepidemiology of human herpesvirus 7 in healthy children and adults in Japan. J Med Virol. 1993;41:319-23.
- [Google Scholar]
- Human herpesviruses 6 and 7 in chronic fatigue syndrome: A case-control study. Clin Infect Dis. 2000;31:48-52.
- [Google Scholar]
- Delayed primary HHV-7 infection and neurologic disease. Pediatrics. 2014;133:e1541-7.
- [Google Scholar]
- Human herpesvirus 6 and 7 in febrile status epilepticus: The FEBSTAT study. Epilepsia. 2012;53:1481-8.
- [Google Scholar]
- Herpetic (non-Cytomegalovirus) retinal infections in patients with the acquired immunodeficiency syndrome. Curr HIV Res. 2013;11:210-9.
- [Google Scholar]
- HHV-8-associated lymphoma: State-of-the-art review. Acta Haematol. 2007;117:129-31.
- [Google Scholar]
- High human herpesvirus 8 (HHV-8) prevalence, clinical correlates and high incidence among recently HIV-1-infected subjects in Sao Paulo, Brazil. PLoS One. 2009;4:e5613.
- [Google Scholar]
- Kaposi's sarcoma in sub-Saharan Africa: A current perspective. Curr Opin Infect Dis. 2010;23:119-23.
- [Google Scholar]
- Seroprevalence and determinants of Kaposi sarcoma-associated human herpesvirus 8 in Indian HIV-infected males. AIDS Res Hum Retroviruses. 2014;30:1192-6.
- [Google Scholar]
- Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474-83.
- [Google Scholar]
- Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: A meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349-59.
- [Google Scholar]
- Meta-analysis of randomized trials on the association of prophylactic acyclovir and HIV-1 viral load in individuals coinfected with herpes simplex virus-2. AIDS. 2011;25:1265-9.
- [Google Scholar]
- The cost-effectiveness of herpes simplex virus-2 suppressive therapy with daily aciclovir for delaying HIV disease progression among HIV-1-infected women in South Africa. Sex Transm Dis. 2011;38:401-9.
- [Google Scholar]
- Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: A randomised placebo-controlled trial. Lancet. 2010;375:824-33.
- [Google Scholar]
- New approaches for quantitating the inhibition of HIV-1 replication by antiviral drugs in vitro and in vivo. Curr Opin Infect Dis. 2009;22:574-82.
- [Google Scholar]
- Episodic therapy for genital herpes in sub-saharan Africa: A pooled analysis from three randomized controlled trials. PLoS One. 2011;6:e22601.
- [Google Scholar]
- Valacyclovir decreases plasma HIV-1 RNA in HSV-2 seronegative individuals: A randomized placebo-controlled crossover trial. Clin Infect Dis. 2015;60:1708-14.
- [Google Scholar]
- Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: Results of a randomized clinical trial. J Infect Dis. 2015;213:551-5.
- [Google Scholar]
- Decreased monocyte activation with daily acyclovir use in HIV-1/HSV-2 coinfected women. Sex Transm Infect. 2015;91:485-8.
- [Google Scholar]
- Maternal valacyclovir and infant Cytomegalovirus acquisition: A randomized controlled trial among HIV-infected women. PLoS One. 2014;9:e87855.
- [Google Scholar]
- A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis. 2013;57:1331-8.
- [Google Scholar]
- Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: A randomized controlled trial. J Infect Dis. 2013;208:1366-74.
- [Google Scholar]
- High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: A randomized crossover trial. J Acquir Immune Defic Syndr. 2013;63:201-8.
- [Google Scholar]
- Effects of valacyclovir on markers of disease progression in postpartum women co-infected with HIV-1 and herpes simplex virus-2. PLoS One. 2012;7:e38622.
- [Google Scholar]
- Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: A randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441-8.
- [Google Scholar]





