Translate this page into:
Immunohistochemical evaluation of tumor hypoxia and angiogenesis: Pathological significance and prognostic role in head and neck squamous cell carcinomas
*Corresponding author: Sramana Mukhopadhyay, Department of Pathology and Lab Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. drsramana@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Soni D, Mukhopadhyay S, Goel G, Kapoor N, Gupta V, Das S. Immunohistochemical evaluation of tumor hypoxia and angiogenesis: Pathological significance and prognostic role in head and neck squamous cell carcinomas. J Lab Physicians. 2024;16:277-85. doi: 10.25259/JLP-2023-9-23-(1969)
Abstract
Objectives:
Tumor hypoxia and angiogenesis have been implicated in therapeutic resistance of head and neck squamous cell carcinomas (HNSCCs). Immunohistochemical evaluation of hypoxia-inducible factor-1 alpha (HIF-1 α), a hypoxia transcription factor, and vascular endothelial growth factor (VEGF), a hypoxia-responsive pro-angiogenic factor, can be exploited for prognostication and guiding treatment intensification or de-escalation decisions in HNSCC patients. The purpose of the study is to evaluate the immunohistochemical expression patterns of HIF-1 α and VEGF and the microvessel density (MVD) for angiogenesis in HNSCC and assess their pathological significance and prognostic role.
Materials and Methods:
In this cross-sectional study, immunohistochemical expression of HIF-1 α, VEGF, and MVD through Cluster of Differentiation (CD31) was evaluated in paraffin-embedded tumor resection tissue of 44 patients with HNSCC. Associations among HIF-1 α, VEGF, and MVD with clinicopathological variables were assessed.
Statistical Analysis:
For assessment of association between HIF-1α, VEGF and MVD by CD 31 immunohistochemical markers and other clinicopathological variables Pearson’s chi-square test and Fisher’s exact tests were used. Analysis of survival was done using Kaplan-Meier statistics. Also, the univariate and multivariate analysis were performed using the Cox proportional hazard regression model for the calculation of hazard ratios.
Results:
Nuclear expression of HIF-1 α showed significant association with MVD (P = 0.007) and cytoplasmic expression of HIF-1 α with histologic grade (P = 0.03). Overexpression of HIF-1 α was more frequent in T3/T4 stage. In addition to cytoplasmic staining, VEGF showed a unique nuclear expression pattern in four cases of advanced disease with nodal metastasis. Logistic regression analysis showed tumors with nuclear overexpression of HIF-1 α to have increased MVD (P = 0.05), and tumors with higher MVD to have a presence of lymphovascular invasion (P = 0.014). Multivariate analysis showed HIF-1 α nuclear overexpression to be significantly associated with decreased survival of patients (P = 0.05).
Conclusions:
Immunohistochemical overexpression of HIF-1 α and MVD quantification can serve as cost-effective tools for prognostication and treatment modification of HNSCC patients in resourcelimited settings.
Keywords
Angiogenesis
CD31
Hypoxia inducible factor-1 alpha
Head and neck squamous cell carcinomas
Vascular endothelial growth factor
INTRODUCTION
Head-and-neck squamous cell carcinoma (HNSCC) is an important cause of carcinoma related morbidity and mortality worldwide. Notably, in India oral cavity carcinoma is a leading cause of cancer among men due to higher prevalence of tobacco chewing and alcohol consumption.[1,2] The mainstay of treatment in HNSCC is surgery with or without radiation and chemotherapy.
Hypoxia and angiogenesis are key tumor microenvironmental factors that have been implicated in conferring radiation resistance and an overall more aggressive biological phenotype in solid malignant tumors.[3,4]
Hypoxia-inducible factor (HIF) is a family of heterodimeric transcription factors that are upregulated in response to hypoxia and regulate hypoxia-driven changes in tumor cells. HIF-1 α is a member of this family.[5] Under normoxic conditions, HIF-1 α subunits are degraded in the cytoplasm, whereas in hypoxia, the degradation is inhibited, as enzymes that modify HIF become inactive. HIF-1 α, then, binds to hypoxia response elements (HREs) present in the nuclear deoxyribonucleic acid and regulates changes in expression of downstream target genes. Vascular endothelial growth factor (VEGF) a hypoxia-responsive gene is a key downstream target of HIF-1 α activation, and induces tumor angiogenesis.[6] Tumor angiogenesis is commonly assessed by intratumoral microvessel density (MVD). Cluster of Differentiation (CD31) is a marker that can selectively detect a glycoprotein of 130kDa in vascular endothelial cells and can aid in assessment of MVD.[7]
Biomarkers that can detect tumor hypoxia and angiogenesis early enough in HNSCCs and predict tumor aggressiveness and its sensitivity to treatment have been a key area of research in recent years. HIF-1 α and VEGF have shown promise in this respect. The immunohistochemical evaluation of these markers associated with clinicopathological data may be a cost-effective method to guide treatment. This is particularly in resource-limited set ups of low- and middle-income group countries.[3,8]
The present study was thus undertaken to study the immunohistochemical expression patterns of these markers and to evaluate their association with known prognostic clinical and histopathological parameters of HNSCC.
MATERIALS AND METHODS
This cross-sectional study conducted by the Departments of Pathology, Otorhinolaryngology, and Radiotherapy, of a tertiary care center in Central India included 44 patients. These patients were operated for HNSCC between January 2020 and December 2021. The clinical details and demographic profile of each patient were recorded. Tumor focality, size, tumor thickness, dimensions of metastatic nodes, any bony, or salivary gland infiltration were noted. Hematoxylin and eosin (H&E) stained slides were studied for tumor histology, grade, depth of invasion, lymphovascular invasion (LVI), perineural invasion (PNI), a worst pattern of invasion (WPOI), tumor stroma ratio, presence of metastatic nodes, extranodal extension, and status of resection margins. Tumor grading and pathological TNM staging were done according to AJCC 8th edition.[9] Surgically resected margins were considered free when tumor was a distance of ≥5 mm involved when <1 mm and close when the distance was 1–5 mm.[10]
Patients recruited for the study were followed up till April 2022. This study was approved by the Institutional Human Ethics Committee for Postgraduate Research (2020/PG/Jan/19).
Immunohistochemical staining
Paraffin-embedded tissue blocks with sufficient tumor were selected for immunohistochemical analysis, and 4–5 microns thickness sections were cut onto positively charged hydrophobic slides (Path-n-situ, Cat#:PS011). Prediluted and ready to use HIF-1 α rabbit monoclonal antibody (EP118, Bio SB, CA-93111, USA), VEGF rabbit monoclonal antibody (Bio SB, CA 93111, USA), CD31 mouse monoclonal antibody (JC/70 A Bio Genex, CA-94538 USA), and p16 (mouse monoclonal Antibody, 16P04, JC2 3 mL predilute RTU, Bio SB, CA-93111 USA) for assessing human papillomavirus (HPV) status were applied. VENTANA Benchmark XT (Ventana Medical Systems Inc. Tucson, Arizona), a fully automated immunostainer, was used to perform immunohistochemistry (IHC) in a semi-automated fashion by manually adding the antibodies.
Tonsillar tissue served as a positive control for HIF-1 α based on the manufacturer’s recommendation. Lymphocytes within the tissue were taken as internal control for HIF-1 α.[11] Blood vessels at the periphery of the tumor tissue sample were taken as internal control for VEGF.
Interpretation
All immunostains were evaluated by three independent observers on an Olympus microscope (BX43F) for percentage of tumor cells staining positive, staining intensity, and cellular localization. For any difference in evaluation, the slides were reviewed in a multiheaded microscope and a consensus was arrived at.
HIF-1 α and VEGF
For HIF-1 α, tumor cells were examined for any cytoplasmic and/or a nuclear staining. Cytoplasmic and nuclear expression were assessed as per protocol of the previous studies.[12-14]
For VEGF, tumor cells were examined for cytoplasmic and/or membranous or any nuclear expression pattern on IHC.
For both the markers, the percentage of tumor cells (0–100%) staining positive was noted, and staining intensity was graded as: 1-weak, 2-moderate, and 3-strong. Expression of HIF-1 α and VEGF was assessed by deriving the H-score (scale: 1–300) using the formula H-score = ΣPi (i + 1), where Pi is the percentage (0–100%) of stained tumor cells at each intensity and i is the intensity: i = 1 (weak), 2 (moderate), and 3 (strong). Separate H-scores were calculated for both nuclear and cytoplasmic staining. These H-scores were categorized quantitatively into low and high groups based on the median value.[15]
MVD
Assessment of MVD was done for quantification of tumor angiogenesis with CD31 IHC. Vessels with a clearly defined lumen or well-defined linear shape were taken into account for counting. Since CD 31 can also stain macrophages, platelets, and immune-poietic cells, single positively stained cells were not considered.[16] MVD was calculated by vascular hotspot method.[7,13,17] At low magnification (×40 and ×100), regions with the highest density of vessels (hot spots) were selected, and the mean number of CD31-positive vessels counted in 3 high-power fields (×200) represented the MVD of that tissue. MVD was dichotomized as low and high on the basis of the respective median values. Images from high-power fields (×200) in the hot spot area were also captured using Leica Microscope (Model Leica DM 750).
P16 IHC was analyzed as per AJCC 8th edition recommendations.[9]
Patients recruited for the study were followed up till April 2022.
Statistical analysis
The data collected were entered in Microsoft Excel and the analysis was carried out in IBM-Statistical Package for the Social Sciences v26 and R v4.0.3. For assessment of association between HIF-1 α, VEGF, and MVD by CD 31 immunohistochemical markers and other clinicopathological variables, Pearson’s Chi-square test and Fisher’s exact tests were used. Analysis of survival was done using Kaplan– Meier statistics. Furthermore, the univariate and multivariate analysis were performed using the Cox proportional hazard regression model for the calculation of hazard ratios. During all the analyses, P-value of equal or ≤0.05 was considered to be statistically significant.
RESULTS
A total of 44 participants were included in this study. The median age was 45 years (age range: 26–73 years) with 75% (n = 33) of study patients being <50 years. Male patients predominated, comprising 36 cases (81.8%), with a male-to-female ratio of 4.5:1.
Buccal mucosa was the most frequent site (63.6%), followed by the tongue (15.9%), alveolus (11.4%), floor of the mouth (4.5%), lower lip (2.3%), and larynx (2.3%). Wide local excision of buccal mucosa (34.09%) was the most common resection procedure received.
On histopathological examination, 61.4% of cases were moderately differentiated (grade-2), 22.7% were well-differentiated (grade-1), and 15.9% were poorly differentiated (grade-3). More than half of the cases had resection margins close to the tumor (56.80%).
LVI was detected in 13 cases (29.5%), PNI in 15 cases (34.1%), and WPOI-5 in 15 cases (34.1%). Majority (34, 77.3%) were stroma-poor tumors.
A pathological tumor stage of pT3 was present for 18 cases (40.91%) followed by 13 cases (29.55%) in stage pT2, 8 cases (18.18%) in stage pT4a, and 5 cases (11.36%) of pT1. Metastatic nodes were present in 20 cases (45.45%). On pathologic prognostic stage grouping, the largest subset of tumors (16, 36.36%) fell into group Stage IV A.
HIF-1 α immunohistochemical expression
HIF-1 α was localized in both the nucleus and cytoplasm. Patchy to diffuse positivity in tumor cells with variable staining intensity was noted. A strong intensity nuclear staining was noted in a majority (36, 81.88%). Cytoplasmic staining was fine, granular to coarse, with strong cytoplasmic staining noted in (9, 20.5%). Well-differentiated squamous cell carcinomas exhibited strong HIF-1 α nuclear and cytoplasmic expression, particularly within the keratin pearls [Figure 1].
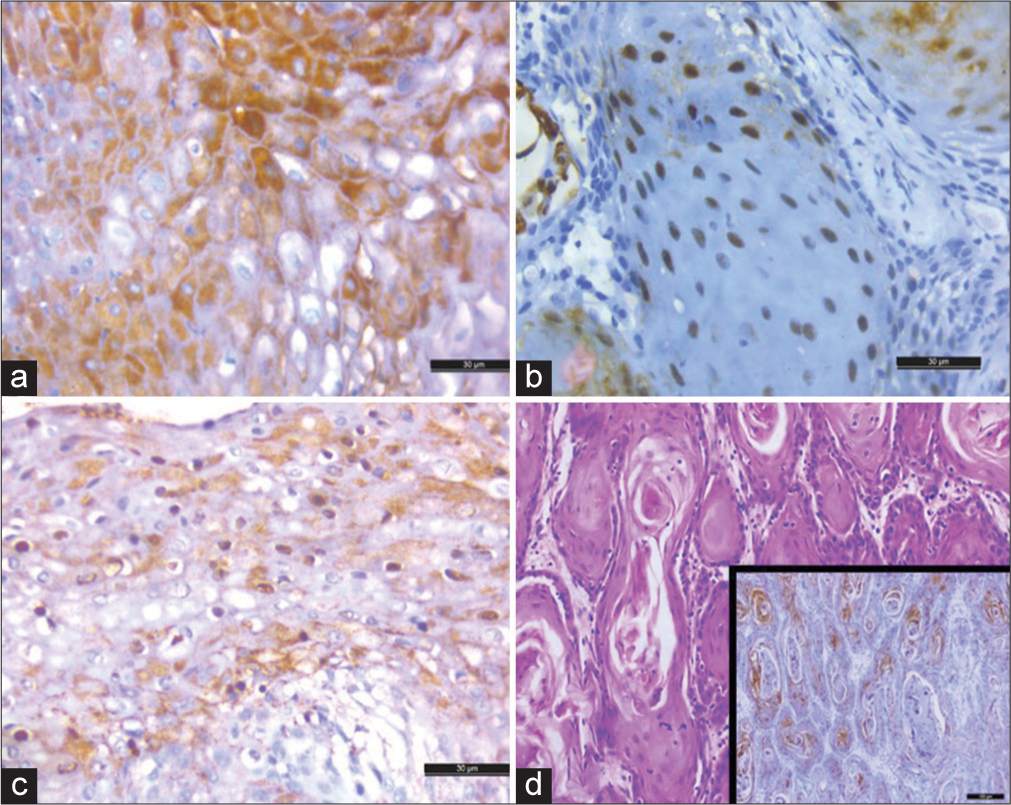
- Hypoxia inducible factor 1 alpha (HIF-1 α) in tumor cells: (a) Cytoplasmic expression (DAB, ×40 objective lens), (b) nuclear expression (DAB, ×40 objective lens), (c) both cytoplasmic and nuclear expression of HIF-1 α (DAB, ×40 objective lens), and (d) strong HIF-1 α immunostaining around keratin pearls in well differentiated tumor (hematoxylin and eosin, ×10 objective lens, inset of DAB). DAB: Diaminobenzidine.
Tumor cells adjacent to blood vessels were negative for HIF-1 α, whereas tumor cells away from blood vessels were strongly nuclear positive [Figure 2].

- (a) Image showing tumor hypoxia in relation to distance from blood vessel (Figure made with BioRender online software), (b) photomicrograph showing positive expression of hypoxia inducible factor (HIF)-1α in tumor cells ~150 µm away from blood vessel (DAB, ×10 objective lens; Red arrow head: Blood vessel; Black arrow head: Tumor cells with no expression for HIF-1α; Yellow arrow heads: Nuclear expression of HIF. DAB: Diaminobenzidine.
Tumor adjacent nondysplastic mucosal epithelium showed nuclear expression of HIF-1 α, in the lower half of the epithelium, except in the basal layer., While the dysplastic mucosal epithelium showed nuclear expression in the lower half of the epithelium,including the basal layer.
HIF-1 α was negative in non-tumoral cells of the stroma except in inflammatory cells, mainly lymphocytes which exhibited strong nuclear staining for HIF-1 α serving as an internal control.
The median H-score for cytoplasmic expression of HIF-1 α was 80 (Range 2–300), with 25 cases (56.8%) of high H-score (>80) and 19 cases (43.2%) with low H-score (<80). The median H-score for nuclear expression of HIF-1 α was 20, with 23 cases (52.2%) having a high H-score and 21 cases (47.7%) with low H-score.
VEGF immunohistochemical expression
Cellular localization of VEGF was observed mainly in the cytoplasm (n = 40, 90.09%), with occasional cases showing additional membranous staining. Staining intensity was variable. Majority (26, 59.1%) showed a weak VEGF expression. Nuclear expression,too was observed, although in only four cases (9.1%), all with higher histologic grades, lymph node metastasis, and belonging to an advanced stage group (stage IVA) [Figure 3]. Tumor adjacent to normal mucosal epithelium was negative for VEGF.
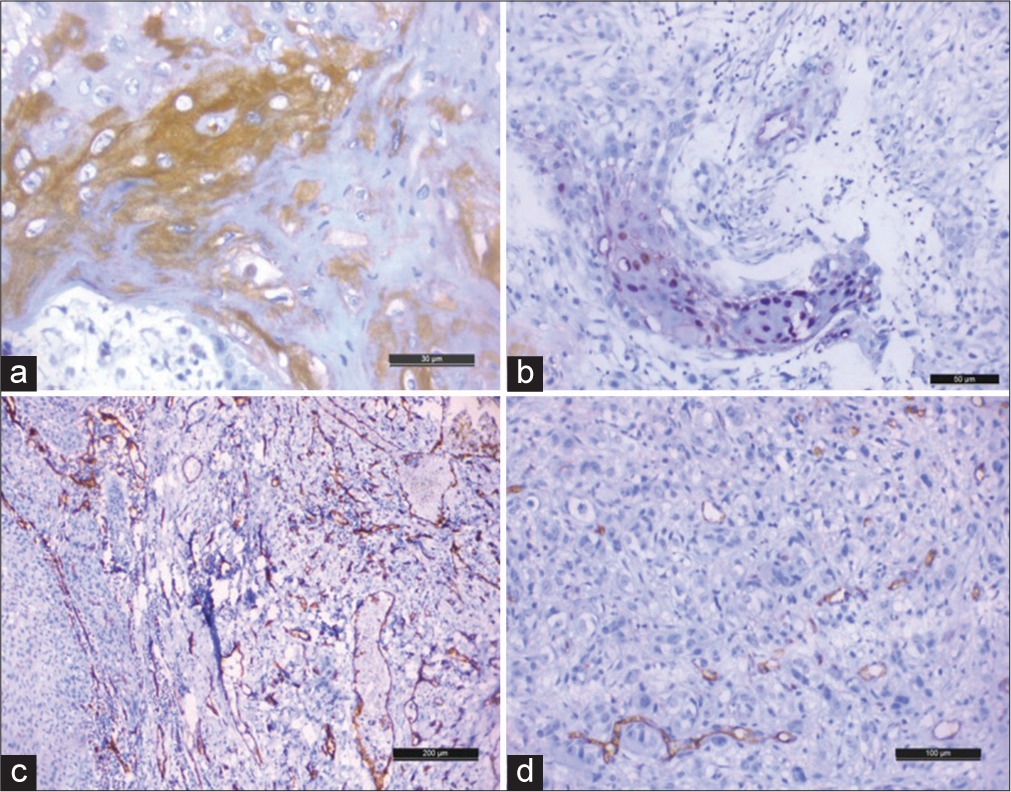
- (a) Strong cytoplasmic expression of vascular endothelial growth factor (VEGF) in tumor cells (DAB, ×40 objective lens), (b) nuclear expression of VEGF in tumor cells (DAB, ×20 objective lens), (c) high microvessel density (MVD) with numerous dilated, tortuous and many slit like vessels highlighted by CD31 (DAB, ×10 objective lens), and (d) low MVD with small slit like vessels highlighted by CD31 (DAB, ×10 objective lens). DAB: Diaminobenzidine.
For quantification of VEGF expression, H-score was calculated and ranged from 0 to 175, with a median value of 20. Of the 44 cases, 25 (56.8%) had high H-scores, while 19 (43.2%) had low H-scores for immunohistochemical expression of VEGF in tumor cells.
MVD
For MVD assessment, the average intratumoral microvessel count ranged from 31 to 96.6. Based on a median cutoff point of 57.95, cases were dichotomized into high and low MVD group. Each category group had 22 cases (50%).
HPV status
Only one case in this study showed positive p16 IHC (>70% diffuse nuclear and cytoplasmic staining) in which the tumor involved the gingiva buccal mucosa.
Survival status/follow-up
Thirteen (29.5%) out of 44 patients had succumbed to the disease, with two dying due to COVID-19-related complications. Thirty-one (70.5%) patients were alive at the time of follow-up.
Associations of HIF-1 α VEGF and MVD
Demographics parameters and tumor-related characteristics did not show any statistically significant association.
Histologic grade of HNSCCs showed a significant association with cytoplasmic expression of HIF-1 α (P = 0.030*) but not with VEGF or MVD.
MVD had a statistically significant association with LVI (P = 0.021*). No significant association was noted for PNI or WPOI.
HIF-1 α overexpression along with higher VEGF scores was observed in pT3/pT4 stage (14, 32%). However lower expression was observed in tumors of pT1/pT2 stage (7, 36.84%).
A statistically significant association with pathologic stage groups III and IV was found with MVD (P = 0.042*).
A significant association was also observed between nuclear HIF-1 α and MVD (P = 0.007*) but not with VEGF [Table 1].
| Characteristic | Low (n, %) | High (n, %) | P-value | n=44 | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|
| Nuclear expression HIF-1α (H-score) | 21 (48) | 23 (52) | |||||
| VEGF | |||||||
| Below median (low) | 8 (38.10) | 11 (47.83) | 0.5 | 44 | — | — | — |
| Above median (high) | 13 (61.90) | 12 (52.17) | 44 | 0.17 | 0.01, 1.85 | 0.2 | |
| MVD | |||||||
| Below median (low) | 15 (34) | 7 (16) | 0.007* | 44 | — | — | — |
| Above median (high) | 6 (14) | 16 (36) | 44 | 26.9 | 1.71, 3,029 | 0.057* | |
| Cytoplasmic expression HIF-1α (H-score) | 19 (43) | 25 (57) | |||||
| Histological grade | |||||||
| Grade 3 | 6 (14) | 1 (2.3) | 0.030* | 44 | — | — | — |
| Grade 2 | 11 (25) | 16 (36) | 44 | 22.8 | 1.47, 1037 | 0.05 | |
| Grade 1 | 2 (4.5) | 8 (18) | 44 | 84.9 | 3.83, 5998 | 0.014* | |
| VEGF | |||||||
| Below median (Low) | 8 (42) | 11 (44) | 0.7 | 44 | — | — | — |
| Above median (High | 11 (58) | 14 (56) | 44 | 0.39 | 0.06, 2.08 | 0.3 | |
| MVD | |||||||
| Below median (Low) | 11 (25) | 11 (25) | 0.4 | 44 | — | — | — |
| Above median (High) | 8 (18) | 14 (32) | 44 | 3.19 | 0.45, 29.5 | 0.3 | |
| MVD | 22 (50) | 22 (50) | |||||
| LVI | 44 | ||||||
| Absent | 12 (54.55) | 19 (86.36) | 0.021* | 44 | — | — | — |
| Present | 10 (45.45) | 3 (13.64) | 44 | 0.1 | 0.01, 0.55 | 0.015* | |
| Pathologic stage group | 44 | ||||||
| Stage 1 and 2 | 3 (13.64) | 9 (40.91) | 0.042* | 44 | — | — | — |
| Stage 3 and 4 | 19 (86.36) | 13 (59.09) | 44 | 0.23 | 0.04, 0.93 | 0.051* |
n (%); Median Fisher’s exact test; Pearson’s Chi-squared test; Wilcoxon rank sum test. OR: Odds ratio, CI: Confidence interval, HIF-1α:Hypoxia inducible factor 1 alpha, VEGF: Vascular endothelial growth factor, MVD: Micro-vessel density, LVI: Lymphovascular invasion. *Significant P-value
The expression of p16 HNSCC showed a skewed distribution as >95% of cases were negative, and no statistically significant associations were observed for p16 expression [Supplementary Table 1].
Logistic regression analysis
A regression analysis revealed higher odds of noting nuclear overexpression of HIF-1 α in cases with high MVD compared to those with low MVD (Odds ratio [OR] = 26.9; 95% confidence interval [CI] = 1.71–3,029; P = 0.057).
Cytoplasmic overexpression of HIF-1 α was observed in tumors with well-differentiated morphology. This observation was statistically significant (OR: 84.9, 95% CI: 3.83–5998, P = 0.014*) when regression analysis was performed,keeping grade-3 tumors as reference. Lack of cytoplasmic overexpression of HIF-1 α was observed in poorly differentiated grade-3 tumors. High MVD and the presence of LVI were associated statistically (P = 0.015*) and showed higher odds with OR = 0.1 with 95% CI ranging from 0.01 to 0.55. The advanced stage of tumor had higher odds of showing high MVD compared to those tumors with early stage (OR: 0.23, 95% CI: 0.04–0.93). These results showed statistically significant associations with P = 0.051*.
Survival analysis
The cumulative survival rate was calculated by the Kaplan– Meier method, and the significance of differences in survival was analyzed by the log-rank test. Mean survival time of patients with low cytoplasmic expression of HIF-1 α was 457.4 (±74.3) days (95% CI: 311.7–603.1), while those with high expression was 345.9 (±33.2) days (95% CI: 280.8– 411.0). Log rank statistics was used, and the Chi-square value of the analysis was 2.824,and P = 0.093 was reported.
The mean survival time of patients with low nuclear expression of HIF-1 α was 455.9 (±64.2) days (95% CI: 330.1– 581.7), while those with high expression was 337.6 (±39.7) days (95% CI: 259.7–415.4). Log rank statistics was used for the analysis, wherein the Chi-square value of the analysis was 2.226 and P-value was reported to be 0.136.
Prognostic significance
On multivariate Cox Proportional Hazards analysis, patients with high nuclear expression of HIF-1 α (above median H-score) showed a trend toward (4.29 times) decreased survival and succumbed to death compared to low nuclear expression of HIF-1 α (Hazard ratio: 4.29, 95% CI: 0.94–19.5, P = 0.059*) [Table 2].
| Predictor | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Cytoplasmic HIF-score | 3.63 (0.82, 16.1) | 0.09 |
| Nuclear HIF-score | 4.29 (0.94, 19.5) | 0.059* |
| MVD | 0.21 (0.04, 1.08) | 0.06 |
| VEGF H score | 0.51 (0.11, 2.31) | 0.4 |
| Histological grade-3 | 14.2 (1.24, 162) | 0.033* |
CI: Confidence interval, HIF: Hypoxia inducible factor, VEGF: Vascular endothelial growth factor, MVD: Micro-vessel density, *Significant P-value.
DISCUSSION
Previous studies have shown tumor hypoxia and angiogenesis to have a bearing on therapeutic resistance, locoregional recurrence, and the metastatic potential of HNSCC.[18] In India, a high burden of cancer cases is attributed to squamous cell cancers of the head and neck region, particularly in the oral cavity, due to the higher prevalence of tobacco chewing. This was noted in the study cohort too, where oral cavity,particularly the buccal mucosa was the most common site of malignancy. Furthermore, there was a rise in the incidence of HNSCC in the young that was also noted in the present study cohort.
These clinical and demographic factors have a peculiar bearing on the treatment outcome of HNSCC patients treated in resource-limited settings of low- and middle-income group countries. The radiation therapy facilities in these countries are often unevenly distributed. Biomarker studies that can either predict treatment responses or guide treatment decisions are, thereby, specifically relevant.
A noteworthy finding in this study was the presence of both cytoplasmic and nuclear expression of HIF-1alpha in all tumors. The cytoplasmic expression was significantly associated with the histological grade (P = 0.030*). Hence, even though a few previous studies have considered only nuclear expression for a positive interpretation that we recommend taking into account both cytoplasmic and nuclear expression.[19-24]
This can be further substantiated by the inherent biology of cancer cells,where a dip in the oxygen concentration in the tumor microenvironment initiates increased intracellular accumulation of HIF-α subunits; Thereby, this translates into cytoplasmic overexpression of HIF-1 α on IHC. On the other hand, nuclear localization of the HIF-α subunit occurs during hypoxia, where the HIF-1 α/ARNT dimer binds to HREs of target genes in the nucleus required for adaptive cellular responses. Tumor cells showing nuclear overexpression for HIF-1 α on IHC may thus be interpreted as frankly hypoxic.
Furthermore, HIF-1 α nuclear overexpression was noted in >60% cases (n = 26) of advanced T stages (T3/T4) of tumor in this study cohort.[25]
The delivery of cancer chemotherapeutics is crucial for radiotherapy and chemotherapy response. Poorly vascularized tumors may hinder drug delivery, while highly vascularized tumors may not fully benefit from hypoxia sensitizers in radiation therapy. Tumor angiogenesis enables growth but may lead to leaky vessels,hindering drug diffusion to tumor cells. VEGF is a key pro-angiogenic factor that regulates tumor angiogenesis.[14] Likewise erstwhile studies, immunohistochemical expression of VEGF was observed in the cytoplasm of tumor cells.[6,13,26-33] However, nuclear staining in addition to cytoplasmic staining, was noted in four cases that belonged to the stage group IVA with metastatic tumor deposits in the lymph nodes. A couple of preceding studies have noted nuclear localization of VEGF (messenger RNA) postulating a nuclear function,especially during hypoxia.[34,35]
Statistically significant associations were not recorded for VEGF with HIF-1 α expression or MVD, similar to a few previous studies.[14,26,30,36-38] To increase the specificity and nonspecific background staining, we used a rabbit monoclonal antibody. This may explain the weak to moderate intensity cytoplasmic staining in a restricted pattern in most of our study cases.
In our study, MVD showed a statistically significant association with the nuclear expression of HIF-1 α. We observed a higher nuclear expression of HIF-1 α cases with high MVD compared to those with low MVD (OR = 6.9, 95% CI = 0.49–3.1 P = 0.057). Although, cytoplasmic staining was noted, it was the nuclear expression of HIF-1 α and not cytoplasmic expression that was associated with angiogenesis. This supports the concept of hypoxia- inducing angiogenesis through nuclear localization and upregulation of the HIF-1 α in tumor cells.[13,30,31,39,40]
Our study is also one of the first to report a significant association between LVI and MVD in HNSCC. Regression analysis also o performed revealed that high MVD and the presence of LVI were associated statistically (P = 0.015*).
We quantified angiogenesis and found MVD significantly associated with the stage of disease (P = 0.042*). On logistic regression analysis, an advanced-stage of tumor showed higher odds of showing high MVD compared to those in the early stage (OR: 0.23, 95% CI: 0.04–0.93, P = 0.051).
Limitations
This was a single institution prospective cross-sectional study with a relatively limited sample size. Due to the skewed distribution of cases with p16 expression, the association between HPV status and hypoxia and angiogenesis markers could not be evaluated, which was another limitation of this study.
CONCLUSIONS
The findings of this study indicate immunohistochemical expression analysis of hypoxia and angiogenesis markers; Notably, HIF-1 α coupled with MVD quantification, may be promising and cost-effective biomarkers that can be utilized for guiding escalation or de-escalation of treatment in HNSCC patients. This will be of great significance particularly in low-to-middle-income group countries, where radiotherapy centers are unevenly distributed and limited resources are present. For positive interpretation of HIF-1 α, we recommend taking into account both nuclear and cytoplasmic staining. Nuclear expression of VEGF was also a unique finding in a few cases of advanced-stage HNSCC with lymph node metastasis. Nuclear localization of VEGF in existing literature has been linked to chronic hypoxia. This is an aspect of VEGF expression on IHC that may be taken up in future studies for further validation.
Ethical approval
Approved by Institutional Human Ethics Committee-PostGraduate Research (IHEC_PGR) Ref number- 2020/PG/Jan/19.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
Dr. Saikat Das is on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Supplementary link
Financial support and sponsorship
Nil.
References
- Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist. 2010;15:994-1001.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate. 2015;75:84-91.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphatic vessel density and vascular endothelial growth factor expression in squamous cell carcinomas of lip and oral cavity: A clinicopathological analysis with immunohistochemistry using antibodies to D2-40, VEGF-C and VEGF-D. Yonago Acta Med. 2013;56:29-37.
- [Google Scholar]
- Micro-vessel density in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2018;275:1845-51.
- [CrossRef] [PubMed] [Google Scholar]
- New prognostic and predictive markers in head and neck tumors. Biomed Res Int. 2016;2016:2850502.
- [CrossRef] [PubMed] [Google Scholar]
- The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93-9.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the Brandwein Gensler risk model in patients of oral cavity squamous cell carcinoma in north India. Head Neck Pathol. 2020;14:616-22.
- [CrossRef] [PubMed] [Google Scholar]
- Overexpression of hypoxia-inducible factor 1a in common human cancers and their metastases. Cancer Res. 1999;59:5830-5.
- [Google Scholar]
- Overexpression of hypoxia-inducible factor 1a indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234-40.
- [Google Scholar]
- Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192-202.
- [CrossRef] [PubMed] [Google Scholar]
- The correlation between HIF-1 alpha and VEGF in oral squamous cell carcinomas: Expression patterns and quantitative immunohistochemical analysis. J Chin Med Assoc. 2018;81:370-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic and predictive significance of nuclear HIF1 a expression in locally advanced HNSCC patients treated with chemoradiation with or without nimotuzumab. Br J Cancer. 2020;123:1757-66.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of microvessel density in tumor and peritumoral area as evaluated by CD31 protein expression and argyrophilic nucleolar organizer region count in endothelial cells in uterine leiomyosarcoma. Sarcoma. 2012;2012:594512.
- [CrossRef] [PubMed] [Google Scholar]
- Meta analysis: HPV and P16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci Rep. 2017;7:16715.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol. 2019;7:4.
- [CrossRef] [PubMed] [Google Scholar]
- Microvascular density and hypoxia-inducible factor in intraepithelial vocal fold lesions. Eur Arch Otorhinolaryngol. 2019;276:1117-25.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of hypoxia-inducible factor-1a: A novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911-6.
- [Google Scholar]
- Overexpression of hypoxia-inducible-factor 1a(HIF-1a) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer. 2003;89:1042-7.
- [CrossRef] [PubMed] [Google Scholar]
- The relation between hypoxia-inducible factor (HIF)-1a and HIF-2a expression with anemia and outcome in surgically treated head and neck cancer. Cancer. 2006;107:757-66.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266-76.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical detection of osteopontin in advanced head-and-neck cancer: Prognostic role and correlation with oxygen electrode measurements, hypoxia-inducible-factor-1a-related markers, and hemoglobin levels. Int J Radiat Oncol Biol Phys. 2006;66:1481-7.
- [CrossRef] [PubMed] [Google Scholar]
- Poor prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinomas that overexpress hypoxia inducible factor-1a. Head Neck. 2016;38:1338-46.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-induced up-regulation of angiogenin, besides VEGF, is related to progression of oral cancer. Oral Oncol. 2012;48:1120-7.
- [CrossRef] [PubMed] [Google Scholar]
- CD31 and VEGF are prognostic biomarkers in early-stage, but not in late-stage, laryngeal squamous cell carcinoma. BMC Cancer. 2018;18:272.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624-30.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular endothelial growth factor expression in untreated and androgen-deprived patients with prostate cancer. Pathol Res Pract. 2005;201:593-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intratumor microvessel density in biopsy specimens predicts local response of hypopharyngeal cancer to radiotherapy. Jpn J Clin Oncol. 2003;33:613-9.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of vascular endothelial growth factor and microvessel density in oral tumorigenesis. J Oral Maxillofac Pathol. 2012;16:22-6.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia inducible factor-1alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res. 2008;28:1659-66.
- [Google Scholar]
- Correlation of hypoxia-inducible factor-1 alpha (HIF-1 a) and vascular endothelial growth factor (VEGF) expressions with clinico-pathological features of oral squamous cell carcinoma (OSCC) Tanta Dent J. 2015;12:S1-14.
- [CrossRef] [Google Scholar]
- Nuclear localization of long-VEGF is associated with hypoxia and tumor angiogenesis. Biochem Biophys Res Commun. 2005;332:271-8.
- [CrossRef] [PubMed] [Google Scholar]
- New horizons for VEGF. Is there a role for nuclear localization? Acta Histochem 2005. ;. ;106:405-11.
- [CrossRef] [PubMed] [Google Scholar]
- VEGF and CD 34: A correlation between tumor angiogenesis and microvessel density-an immunohistochemical study. J Oral Maxillofac Pathol. 2013;17:367-73.
- [CrossRef] [PubMed] [Google Scholar]
- Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94-101.
- [CrossRef] [PubMed] [Google Scholar]
- Angiogenic factors in squamous cell carcinoma of the oral cavity: Do they have prognostic relevance? J Craniomaxillofac Surg. 2004;32:176-81.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia inducible factor 1a and hypoxia inducible factor 2a play distinct and functionally overlapping roles in oral squamous cell carcinoma. Clin Cancer Res. 2010;16:4732-41.
- [CrossRef] [PubMed] [Google Scholar]
- HIF 1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer. 2005;5:84.
- [CrossRef] [PubMed] [Google Scholar]






