Translate this page into:
In vitro cost-effective methods to detect carbapenemases in Enterobacteriaceae
Address for correspondence: Dr. Varsha Gupta, Department of Microbiology, Government Medical College Hospital, Sector 32-B, Chandigarh - 160 030, India. E-mail: varshagupta_99@yahoomail.com
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The rise in carbapenemases-producing organisms has challenged the scientific community. Infections caused by these bacteria have limited treatment options. There are various types such as Klebsiella pneumoniae carbapenemase (Ambler class A), metallo-beta-lactamases of VIM-type, IMP-type, NDM-type (Ambler class B), and OXA-48-types (Ambler class D). An efficient strategy for detection of carbapenemase producers is important to determine the appropriate therapeutic modalities. In this study, four methods - Carba NP test, modified Carba NP (MCNP) test, carbapenem inactivation method (CIM) test, and Rapidec Carba NP kit test were evaluated. We evaluated an in-house MCNP test to detect carbapenemase production using a single protocol which gave reliable results. Furthermore, CIM using routine antibiotic discs gives good results. Both these tests were found to be cost-effective.
Keywords
Carbapenemases
Enterobacteriaceae
imipenem
Introduction
The rise in carbapenemase-producing organisms has challenged the scientific community. Infections caused by these bacteria have limited treatment options. Carbapenemase producers are mainly identified as Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter species and reported mostly in hospital settings.[1] Carbapenemases are specific beta-lactamases with the ability to hydrolyze carbapenems. Various enzymes such as K. pneumoniae carbapenemase (Ambler class A), metallo-beta-lactamases of VIM-type, IMP-type, and NDM-type (Ambler class B), and OXA-48-types (Ambler class D) are commonly responsible for this type of resistance in Gram-negative bacteria.[2] An efficient strategy for detection of carbapenemase producers is important to determine the appropriate therapeutic modalities.[2] The Clinical and Laboratory Standards Institute recommended the use of modified Hodge test for carbapenemase detection in Enterobacteriaceae but strains that produce both extended spectrum beta-lactamases or AmpC enzymes coupled with porin loss may show false positive results and some isolates producing NDM-type carbapenemase may show false negative results.[3] Carba NP test has been developed by CLSI for rapid identification of carbapenemase production in Enterobacteriaceae as well as for P. aeruginosa and Acinetobacter spp. that are nonsusceptible to one or more carbapenems.[3] This biochemical test, applicable to isolated bacterial colonies, is based on in vitro hydrolysis of the carbapenem, i.e., imipenem. Hydrolysis of imipenem is detected by a change in the pH value of the indicator (red to yellow/orange). This test is 100% sensitive and specific as are molecular techniques. In addition, it does not require any specific equipment.[4] Besides this test, some modifications of this test and commercial kits have also become available recently for detection of carbapenemases. In this study, four methods - Carba NP test, MCNP test, carbapenem inactivation method (CIM) test, and Rapidec Carba NP kit were evaluated. The included tests are based on biochemical detection of the hydrolysis of the beta-lactam ring of a carbapenem that is followed by color change of a pH indicator.
Purpose
The purpose of this study was to detect carbapenemase production in Enterobacteriaceae, Pseudomonas, and Acinetobacter species using a single protocol with rapid results, good reliability, and speed.
Materials and Methods
This study included a total of 75 isolates of Enterobacteriaceae family, of which 42 isolates were K. pneumoniae, 18 isolates were E. coli. Further eight isolates were Acinetobacter cbc and seven isolates were P. aeruginosa to detect carbapenemase production by various methods.
Various tests performed for the detection of carbapenemases
Carba NP test
Materials and reagents required
-
Clinical laboratory reagent water (CLRW)
-
Imipenem reference standard powder (Sigma-Aldrich Chemie GmbH 10160, Germany)
-
Commercially available B-PER bacterial protein extraction reagent in Tris-HCl buffer, pH 7.4 Product #78248 (Thermo Scientific, 3747 N. Meridian Road, Rockford, IL 61101 USA)
-
Zinc sulfate heptahydrate (Hi Media Laboratories Pvt. Ltd. Works, Mumbai, India)
-
Phenol red powder (Hi Media Laboratories Pvt. Ltd. Works, Mumbai, India)
-
1N NaOH solution
-
10% HCl solution
-
Microcentrifuge tubes 1.5 ml, clear
-
1 μl inoculation loops
-
Containers to store prepared solutions.
Preparation of reagents
-
10 mM zinc sulfate heptahydrate solution:
-
Weigh out 1.4 g ZnSO47H2O
-
Add to 500 ml CLRW
-
Mix
-
Store at room temperature.
-
Expiration: 1 year or not to exceed expiration of individual component.
-
0.5% phenol red solution:
-
Weigh out 1.25 g phenol red powder
-
Add to 250 ml CLRW
-
Mix
-
Store at room temperature.
-
Expiration: 1 year or not to exceed expiry of individual components.
Note: This solution does not remain in solution. Mix well before use.
-
0.1N sodium hydroxide solution:
-
Add 20 ml 1 N NaOH to 180 ml CLRW
-
Store at room temperature.
-
Expiration: 1 year or not to exceed expiry of individual components.
-
Carba NP solution A:
-
In a 25–50 ml beaker, add 2 ml 0.5% phenol red solution to 16.6 ml CLRW
-
Add 180 μl of 10 mM zinc sulfate solution
-
Adjust pH to 7.8 ± 0.1 with 0.1 N NaOH solution if pH is too high
-
Store at 4–8°C in small vial or bottle and protect from prolonged light exposure.
-
Expiration: 2 weeks or not to exceed expiration of individual components (solution should remain red or red-orange; do not use if solution turns any other color).
-
Carba NP solution B (solution A + 6 mg/ml imipenem powder):
-
Determine the amount of solution B needed, allowing 100 μl per tube for each patient, quality control (QC) strain, and an uninoculated reagent control
-
Weigh out approximately 10–20 mg of imipenem powder.
-
Note: it is advisable to weigh out at least 10 mg of powder. Suppose we have weighed 12 mg powder, divide the actual weight by 6 to determine the amount (in ml) of solution A to be added to the powder. As the concentration required for test is 6 mg/ml.
Example: 12 mg of imipenem/6 = 2 ml of solution A, which is sufficient for 20 tubes.
-
Store at 4–8°C for up to 3 days.
Quality control
Control strains included for routine laboratory carbapenem susceptible strains are used to rule out or minimize false positivity and to evaluate the specificity of the test methodology.
QC strains are also used as:
-
K. pneumoniae ATCC BAA-1705-MHT positive
-
K. pneumoniae ATCC BAA-1706-MHT negative.
Test procedure
-
Label two microcentrifuge tubes (one “a” and one “b”) for each patient isolate, QC organism, and uninoculated reagent control
-
Add 100 μl of bacterial protein extraction reagent to each tube
-
For each isolate to be tested, emulsify a 1 μl loopful of bacteria from an overnight growth culture in both tubes “a” and “b”
-
Vortex each tube for 5 s (uninoculated reagent control tubes should contain only bacterial protein extraction reagent, no organism)
-
Add 100 μl of Solution A to tube “a”
-
Add 100 μl of solution B to tube “b”
-
Vortex tubes well
-
Incubate at 35°C ± 2°C for up to 2 h
-
Isolate that demonstrate positive results before 2 h can be reported as carbapenemase producers.
Note: Do not use growth from selective media or plates containing antibiotics or other agents that select for certain bacteria.
Test interpretation
-
Read uninoculated reagent control tubes “a” and “b”
-
Both tubes must be red or red-orange.
-
-
Read inoculated tube “a”
-
Tube “a” must be red or red-orange
-
If tube “a” is any other color, test is invalid.
-
-
Read inoculated tube “b”

- Inoculated tubes (a) showing red color

- Inoculated tubes (b) showing yellow color positive for carbapenemase production
Reporting
Report positive if color changes From red color to yellow color in test tube.
Modified Carba NP test
Materials and reagent required
-
In MCNP method, the therapeutic intravenous (i.v.) imipenem/cilastatin with imipenem IP equivalent to 500 mg powder is used which is used commercially for patient use
-
Lysis buffer used is 0.02% cetyltrimethylammonium bromide (CTAB - Sigma-Aldrich Chemie, GmbH, Germany)
-
pH should be below 7.8
-
No centrifugation and incubation required.
In house modification of the method
In MCNP method, the original publication used imipenem/cilastatin powder with concentration of 6 mg/ml and pH <7.8; however, in our modification, we have increased the concentration of imipenem/cilastatin powder to 8 mg/ml and pH is adjusted to 7.5.
Test procedure
-
Label two tubes for one strain tube “a” and tube “b”
-
Tube “a” as test tube and tube “b” as negative control tube
-
Add 200 μl of 0.02% CTAB (lysis buffer) to each tube
-
Add 100 μl bacterial suspension to each tube
-
Add 100 μl diluted phenol red solution, pH <7.5 to negative control tube “b”
-
Add 100 μl diluted phenol red solution supplemented with imipenem/cilastatin (concentration 8mg/ml) in tube “a”.
-
Vortex tube “a” and tube “b”
-
Incubate at 37°C for 2 h.
Test interpretation
Test tube “a” versus negative control tube “b” and yellow-orange color versus red color is considered as positive for carbapenemases production.
Note: If color does not change to yellow or orange, it should be incubated for 2 h to declare it negative. If color still remains red, then label it as negative for the test [Figure 3].

- Five red color tubes (b) as ‘control’ and next five yellow color tubes (a) as ‘positive’ for carbapenemase production
Carbapenem inactivation method
Material and reagents required
-
Mueller-Hinton agar (MHA) or blood agar plates
-
Distilled water
-
10 μg of meropenem disk (Hi Media Laboratories Pvt. Ltd. Works, Mumbai, India)
-
Control strain of E. coli ATCC – 25922.
Test procedure
-
Add 10 μl inoculation loopful of culture of tested strain in 400 μl distilled water
-
Put 10 μg of meropenem disk into suspension
-
Incubate it for 2 h at 35°C
-
Treated meropenem disk is placed on MHA plates inoculated with ATCC E. coli – 25922 strain as lawn culture
-
Incubate it for 8–10 hours at 37°C [Figure 4].

- Carbapenem inactivation method, showing test strain was resistant on treated meropenem disk, interpreted as positive for carbapenemase production
Interpretation of result
-
If test strain is meropenem resistant, i.e., no zone of inhibition – positive for carbapenemase production and meropenem is hydrolyzed
-
If test strain is sensitive to meropenem – negative for carbapenemase production showing zone of inhibition by meropenem.
Rapidec Carba NP kit test
Material and reagents required
-
Rapidec test kit (BioMerieux SA, France)
-
Test strains
-
10–100 μl pipette
-
Sterile tips.
Test procedure as suggested by manufacturers
-
Step 1: Add 100 μl suspension given with kit in well “a,” “b,” “c” for rehydration of well for 5–10 min with lid covered at room temperature
-
Step 2: Mix the content of well “b” with stirrer
-
Step 3: Add bacterial colonies into well “c” with same turbidity of well “b” and then cover with lid for 30 min at room temperature
-
Step 4: Transfer 25 μl from well “c” to both well “d” and well “e”(well “d” is control well and well “e” is test well)
-
Step 5: Transfer 25 μl from well “a” to both well “d” and well “e”
-
Step 6: Cover the lid and incubate it for 30 min at 37°C [Figure 5].
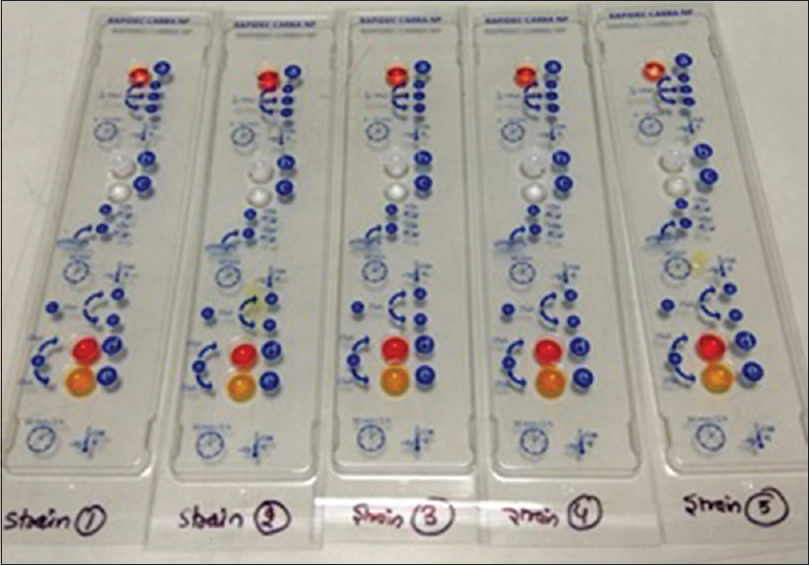
- Rapidec Carba NP test kit, depicting red color in well “d” and yellow color in well “e,” interpreted as positive for carbapenemase production
Test interpretation
-
First reading should be taken after 30 min. If positive for carbapenemase production, well “d” changes to red color and well “e” yellow to orange color
-
For negative result second reading taken after 30 min.
Results
The detection of carbapenemases was done in 75 isolates, of which 42 isolates were K. pneumoniae, 18 isolates were E. coli, 8 isolates were Acinetobacter cbc, and 7 isolates were P. aeruginosa [Table 1].

The results of the study showed that all 75 strains which were positive by Carba NP and Rapidec Carba NP test kit but were coming negative using original MCNP test in which 6 mg/ml concentration of imipenem/cilastatin powder and phenol red with pH <7.8 was being used. In our modification, when we increased the concentration of powder from 6mg/ml to 8mg/ml and reduced the pH of phenol red solution to 7.5, all the results came out to be positive [Figure 3].
Furthermore, in CIM, routine Hi-Media Meropenem discs (10 μg) instead of expensive Oxoid disks performed well and gave 100% results [Figure 4].[6]
Discussion
The detection of carbapenemases is of importance to prescribe an effective antibiotic for the patients having infection with carbapenem-resistant Gram-negative bacteria. Recently, CLSI has introduced a method to detect carbapenemase production based on hydrolysis of the beta-lactam ring of a carbapenem. In developing countries for routine clinical laboratories, a method has to be developed which is easy to perform and cost-effective in determining carbapenemases. Four methods were evaluated in this study. In Carba NP method, the powder and reagents require extemporaneous preparation; however, in MCNP test, the reagent prepared can be stored at 4°C for up to 24 h.[7] In Carba NP test, imipenem reference powder (Sigma) is required which is very expensive while modified test requires therapeutic imipenem/cilastatin powder which is easily available commercially.[7] In our modification, we have used PRIMAPEN powder from NEON LABS. Further in comparison to Carba NP test, in the modified version, no centrifugation and incubation step is required, so the results are available readily. Furthermore, we can modify amount of imipenem and pH of the powder available at our center accordingly. Further, cost-cutting is of help a great extent as expensive reagents are being replaced by reagents easily available from hospital pharmacy and are less costly. The advantages of the MCNP test are the detection of different carbapenemase types from Enterobacteriaceae, Pseudomonas, and Acinetobacter species using a single protocol as well as the short time to results that are available instantly; but to rule out negative results, the tubes can be kept until 2 h.[5] In CIM, the original article used expensive Oxoid meropenem disk (10 μg).[6] While we have used meropenem disk (10 μg) from Himedia laboratories which is relatively cheaper and gave good results. Finally, Rapidec Carba NP test kits (BioMerieux) are expensive compared to CIM and MCNP test. The cost of a single test by Rapidec Carba NP kit comes to about 300 rupees.
Conclusion
To conclude the study, for routine purposes in microbiology laboratories for detection of carbapenemases in Enterobacteriaceae, P. aeruginosa, and Acinetobacter spp., either we can use carbapenem inactivation method or perform an in-house standardized MCNP test which would reduce the cost of the detection of carbapenemases. Further, this MCNP test can be modified in microtiter plates so that for bigger laboratories with big sample size simultaneously, large number of test strains with controls can be put on the same plate thus making it still more cheaper and cost-effective. The limitation of our study is that molecular confirmation of the various carbapenemase genes could not be done, however, that is required for epidemiological purposes only.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2013;68:487-9.
- [Google Scholar]
- 2016 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA
- Rapid detection of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis. 2012;18:1503-7.
- [Google Scholar]
- Rapid identification of carbapenemase-producing Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumannii using a modified Carba NP test. New Microbes New Infect. 2015;7:89-93.
- [Google Scholar]
- The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One. 2015;10:e0123690.
- [Google Scholar]
- Comparison of the Carba NP, Modified Carba NP, and Updated Rosco Neo-Rapid Carb Kit tests for carbapenemase detection. J Clin Microbiol. 2015;53:3539-42.
- [Google Scholar]





