Translate this page into:
Induced Sputum Nitrite Levels Correlate with Clinical Asthma Parameters in Children Aged 7–18 Years with Mild to Moderate Persistent Asthma
Address for correspondence: Dr. Devki Nandan, E-mail: devkinandan2002@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Purpose:
The objective of this study is to measure levels of nitrites in induced sputum in children with asthma and correlate it with clinical asthma parameters.
Method:
This prospective observational study was done in PGIMER, Dr. Ram Manohar Lohia Hospital, New Delhi, on 91 children aged 7-18 years with mild and moderate persistent asthma. Patients were specifically evaluated for five clinical parameters of asthma (i.e. Days of acute exacerbations, use of salbutamol as rescue medication, emergency visits, nights with cough, days of school absence) and induced sputum nitrite levels was done at the time of enrollment and 3 months after treatment with inhaled budesonide.
Results:
The mean age of subjects was 10.79 ± 2.563yrs. Six (6.59%) patients were not able to perform induced sputum, eighty five (93.41%) patients were suitable for data analysis. There was significant reduction in sputum nitrite levels from 33.42 ± 22.04nmol/ml at enrollment to 11.72 ± 5.61 nmol/ml (P < 0.0005) after 3 months of inhaled budesonide therapy. Significant positive correlation was found between reduction in sputum nitrite level and control of asthma symptoms: Days of acute exacerbations(r value = 0.548, P value = 0.0001), Days of salbutamol use as rescue medication (r value = 0.431, P value =< 0.0001), Number of emergency visits(r value = 0.414, P value = 0.0001), Nights with cough (r value = 0.259, P value = 0.0169), Days of school absence(r value = 0.411, P value = 0.0001). Sputum nitrite levels were significantly higher in moderate persistent asthmatics as compared to mild at the time of enrollment (P < 0.0005), which shows that induced sputum nitrite levels correlate with asthma severity.
Conclusions:
This study confirms that nitrites in induced sputum correlate well with clinical asthma parameters and asthma severity in children and is a simple, non invasive, and cheap method which can be used as a parameter for monitoring of asthma.
Keywords
Asthma
clinical parameters
induced sputum
inhaled budesonide
nitrites
INTRODUCTION
Asthma is one of the most prevalent chronic respiratory disease with a prevalence ranging from 1% to 16% of the population in different countries[1] and 11.6% in school going children in Delhi.[2] Suppression of airway inflammation and control of asthma by inhaled steroid therapy remains an important goal of asthma therapy.
Forced expiratory volume in the 1st s (FEV1) is generally recommended as the most valuable lung function outcome by asthma guidelines,[3] however, FEV1 and the severity of asthma in children does not correlate.[4] With treatment, FEV1 normalizes and symptoms get controlled but airway inflammation and even remodeling features persist in asthmatic children and adults,[56] suggesting that FEV1 measurements is not a marker of ongoing airway inflammation and hence sub-clinical disease.
Direct methods such as bronchoscopic biopsies and bronchoalveolar lavage fluid examination are specific and sensitive but are invasive methods to be used in the analysis of airway inflammation in children.[78910] Cellular quantification in sputum samples[1112] and exhaled nitric oxide (eNO) are direct noninvasive methods which are shown to be accurate markers of asthmatic airway inflammation.[13] Sputum induction with hypertonic saline is a safe and easy technique, and analysis of results are similar to results of secretions obtained through the bronchial wash and bronchoalveolar lavage.[14]
eNO is a test which is sensitive and specific as a noninvasive marker of allergic airway inflammation[15161718192021] however, it is not an affordable and accessible method for developing countries. Nitric oxide (NO) metabolites, for example, nitrates and nitrites, in induced sputum have been evaluated, high levels of which have been reported in adults and school children with asthma in comparison to controls.[2223] However, correlation of nitrites in induced sputum with clinical asthma parameters has not been fully studied.
Therefore, the present longitudinal study was planned with the aim of assessing if the levels of nitrite in induced sputum decreases after inhaled budesonide therapy in asthmatic school children and correlates with clinical asthma parameters.
METHODOLOGY
This study was a prospective observational study conducted between November 2013 and December 2014 in Department of Pediatrics, PGIMER and Dr. Ram Manohar Lohia Hospital, which is a Tertiary Care Center in New Delhi. Ninety-one cases of mild to moderate persistent asthma aged 7–18 years were included. Patients attending pediatric chest clinic were screened for asthma and were diagnosed by history and spirometry (Vitalograph Mod-6600): FEV1 >60% and FEV1 >12% reversibility after 10 min of administration of 400 μg of salbutamol metered dose inhaler through valved spacer.
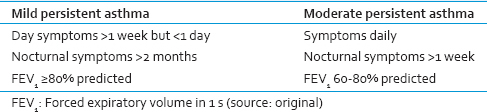
A standardized questionnaire with demographic, anthropometric characteristics, and clinical asthma parameters during the last year (days of acute exacerbations, days with salbutamol used as rescue medication, number of emergency visits, nights with cough, and days with school absence due to asthma) was answered by the child and his/her parents.[24] Clinical severity of asthma was classified according to Global Initiative for Asthma guidelines [Table 1].[25] Children who have received inhaled corticosteroid, nasal steroids or leukotriene modifying agents in previous 2 months and systemic steroids in last 10 days,[8] children having respiratory tract infection 4 weeks prior to study, other chronic respiratory illness, children with major systemic illness, active tobacco consumption, those who are not able to use inhaler with spacer or perform spirometry, and those who refused to give consent were excluded from the study.
Induced sputum was then collected. Patients then received inhaled budesonide at a mean dose of 400 mcg/day delivered by metered-dose inhalers with a valved spacer device[3] and inhaled salbutamol by metered-dose inhaler as needed. Adherence to medication and clinical parameters were checked telephonically on weekly basis and follow-up in pediatric chest clinic was done monthly. After completion of 3 months of inhaled budesonide therapy clinical assessment, spirometry and sputum induction was repeated.
Method of sputum induction
Patients were asked to come early morning for sputum induction. Patients were kept nil per oral 2 h before the sputum induction and not eat lettuce, Chinese food nor drink carbonated beverages 12 h prior. Patients were asked to rinse mouth and blow nose to minimize contamination with saliva and postnasal drip.[24] After a premedication with 400 μg of salbutamol metered dose inhaler via valved spacer, an inhalation with 4 ml of 3% saline solution in aerosol generated by a ultrasonic nebulizer (Multisonic LS290) was delivered via a face mask for a maximum of 30 min. Patients were encouraged to cough deeply and expectorate sputum every 5 min during saline inhalation until adequate sample was obtained (1.5 ml). The sputum sample was collected in a sterile plastic container.[26] FEV1 was repeated after induction and if it fell below 20% of first value saline inhalation was discontinued and the child was required to wait until it returned to baseline. The sputum samples were stored at 4° C for not more than 2 h before further processing.[27]
Sputum processing
Sputum sample was diluted with equal volume of phosphate buffered saline containing 10 mmol/l of dithiothreitol in an equal volume, gently vortexed at room temperature and centrifuged for 10 min. The supernatant was stored at −7°C for subsequent Griess assay.[27] Griess assay was performed for all the samples at the end of the study as a single batch.
Nitrite assay
It was performed by using an NO Colorimetric Assay Kit (MBL Catalog No. JM-K262-200) which works on the principle of Griess reaction (Costing Rs. 200 per sample).
Principle of Griess reaction
Griess reaction relies on a simple colorimetric reaction between nitrites, sulphanilamide and N-(1-naphthyl) ethylenediamide to produce a pink/magenta azo-product with a maximum absorbance at 543 nm.
Method of sputum nitrite measurement
85 μl of sample supernatant was added to each well to which 5 μl of enhancer was added. The plate was incubated for 10 min at room temperature. Greiss reagent1 (0.2% sulfanilamide dissolved in 5% phosphoric acid) and Griess reagent 2 (0.2% naphthylethylene-diamine-dihydrochloride) was added to each well 50 μl each. This was incubated for 10 min at room temperature for color development. Absorbance was read at 540 nm using a plate reader. Absorbance was plotted as a function of nitrite concentration in nmol.
Nitrite concentration was measured by using the formula: Nitrite concentration = (sample absorbed/slope of the standard curve)/85.
Results were expressed in nmol/L.
Ethical clearance
Ethical clearance was taken from Institutional Ethics Committee vide reference number 10280 dated November 11, 2013.
Statistical analysis
Data were analyzed using IBM corp. Released 2012. IBM SPSS stastitics for windows version 21.0, Armonk, NY:IBM corp. Continuous variables were presented as mean ± standard deviation and median ± interquartile range and categorical variables were presented as a percentage. Continuous variables were tested by Mann–Whitney U-test for nonparametric data and by independent t-test for parametric data. Dichotomous variables were tested by Fisher's exact test or Chi-square test. For correlation between different variables, Spearman's correlation was used. P < 0.05 was taken as significant.
RESULTS
In the present study, a total of 91 children aged between 7 and 18 years were enrolled. The study group comprised of all newly diagnosed cases of mild to moderate persistent asthma who met the inclusion criteria. These patients were given treatment with inhaled budesonide at the mean dose of 400 μg and were followed for 3 months. Induced sputum was done before initiation of budesonide therapy and after 3 months of inhaled budesonide therapy. Six children (6.59%) were not able to perform induced sputum. Hence, only 85 (93.41%) patients were suitable for data analysis. Nitrite levels sputum were measured. Furthermore, clinical parameters of asthma (days of acute exacerbation, days of salbutamol use, number of emergency department visits, number of nights with cough, and days of school absence) were enquired at the time of enrollment and then telephonically on weekly basis from the patient and on monthly visits. FEV1 levels were measured at the time of asthma diagnosis and after 3 months of inhaled budesonide therapy.
None of the patients suffered any acute side effects after sputum induction. Average time taken to produce an adequate sample in patients was 10.65 ± 1.69 min.
Sixty-four cases (70.37%) were classified as mild whereas 27 (29.7%) were moderate persistent asthmatics. Demographic profile of the subjects were noted [Table 2]. The levels of induced sputum nitrite were high at the time of enrollment, and the values were more in moderate than mild persistent asthmatics. The reference value was taken from an Indian study done by Kumar et al., where controls were used.[26]

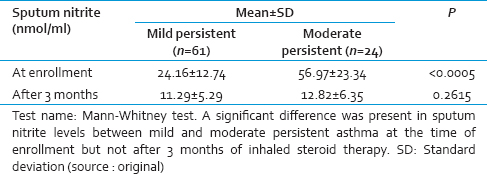
There was a significant reduction in sputum nitrite levels after 3 months of inhaled budesonide therapy in both mild and moderate persistent asthmatics (<0.0005) [Tables 3 and 4].

Significant positive correlation was found between reductions in sputum nitrite levels with control of clinical asthma parameters [Table 5]. There was a significant negative correlation between FEV1 percentage predicted with sputum nitrite levels [Table 6].


DISCUSSION
FEV1 is generally recommended as the most valuable lung function outcome by asthma guidelines,[3] however, FEV1 and the severity of asthma in children does not correlate.[428] With treatment, FEV1 normalizes, and symptoms get controlled, but airway inflammation and even remodeling features persist in asthmatic children and adults,[56] suggesting that FEV1 measurements is not a marker of ongoing airway inflammation and hence sub-clinical disease. Various methods used for assessing airway inflammation have been studied including invasive techniques such as bronchoscopic biopsies and bronchoalveolar lavage fluid examination[78910] and noninvasive ones such as sputum cytologic analysis,[2930] eNO[21] and NO metabolites[2223] in induced sputum. Of these, induced sputum nitrite levels are an easy, nonexpensive and noninvasive method of assessment of airway inflammation. However, correlation of nitrites in induced sputum with clinical asthma parameters has not been fully studied.
Few studies in adults and fewer in children have been conducted in past using nitrate metabolites as a marker of airway inflammation and have studied their use in monitoring of asthma patients. To the best of our knowledge, there is no Indian study which has studied the correlation between induced sputum nitrite levels and clinical asthma parameters in the pediatric population. There is only one study worldwide which has studied this correlation.[24]
In our study, we found that induced sputum nitrite levels were high in cases at enrollment, and there was statistically significant reduction in this parameter after 3 months of inhaled budesonide therapy [Tables 3 and 4]. Mean levels of sputum nitrite was 33.42 ± 22.04 nmol/ml at the time of enrollment which reduced significantly to value of 11.72 ± 5.61 nmol/ml (P < 0.0005) after 3 months of inhaled budesonide therapy. Reduction in sputum nitrite levels after treatment with inhaled corticosteroids were seen in studies conducted previously[22242627] however, the sample size in these studies were small.
In a study conducted by Kumar et al.[26] the levels of induced sputum nitrite continued to be higher than controls even after 6 weeks of anti-inflammatory therapy while nitrite levels were similar to controls in the study done by Castro-Rodriguez et al., at the end of study who gave treatment for 3 months. Hence, we have given therapy for 3 months.
A significant positive correlation was found between the reduction in sputum nitrite levels with the improvement in clinical asthma parameters (i.e., days of acute exacerbations, use of salbutamol as rescue medication, emergency visits, nights with cough, and days of school absence) after 3 months of treatment [Table 5]. Similar results were found in a recent study conducted by Castro-Rodriguez et al.[24] However, in our study number of cases who completed the study were 85 as compared to the latter where it was 62.
In the present study, patients were treated with budesonide for 3 months with a metered dose inhaler with a valved spacer which is an advantage over other studies[2426] who have not used valved devices.[3]
On subgroup analysis of these patients we found that children with moderate persistent asthma had significantly higher values of sputum nitrite levels than children with mild persistent asthma at the time of enrollment (56.97 ± 23.34 nmol/ml vs. 24.16 ± 12.74 nmol/ml, P < 0.0005) but the levels were almost similar at the end of treatment (12.82 ± 6.35 nmol/ml vs. 11.29 ± 5.29 nmol/ml, P = 0.2615). This concludes that induced sputum nitrite levels correlate well with severity of asthma. Similarly, nitrite levels were found to be higher with increasing asthma severity at enrollment in other studies.[222627] This is in contrast with a study conducted by Castro-Rodriguez et al.[24] in 2014, who found no significant difference in median levels of sputum nitrite in mild and moderate persistent asthma children both at the time of admission (34.2 nmol/ml vs. 39.4 nmol/ml, P = 0.36) and after 3 months of inhaled beclomethasone therapy (10.9 nmol/ml vs. 11.4 nmol/ml, P = 0.56). The author has not given any explanation to this variation.
We found that reduction in induced sputum nitrite levels had a significant positive correlation with the improvement of clinical asthma parameters acute exacerbations (r = 0.548, P < 0.0001); use of salbutamol (r = 0.431, P < 0.0001); emergency visits (r = 0.414, P = 0.0001); school absence (r = 0.411, P = 0.0001); and nights with cough (r = 0.259, P = 0.0169) after 3 months of treatment with budesonide metered dose inhaler in children with mild and moderate persistent asthma. Days of acute exacerbations, salbutamol use, emergency visits, and school absence have a more significant correlation with sputum nitrite levels than nights with a cough. A similar reduction was seen in a study done by Castro-Rodriguez et al.[24] However, in their study a negative correlation was seen between sputum nitrite levels and nights with a cough (r = −0.018, P = 0.9) which was not significant.
We found that there was a significant negative correlation between change in sputum nitrite levels and FEV1 after 3 months of inhaled steroid therapy in children with mild and moderate persistent asthma. No study until the date has been done to study the correlation of induced sputum nitrite levels with FEV1 in asthmatic children. However, few adult studies have shown similar results.[3132]
Nitrite in induced sputum is an upcoming area of research in asthmatic children. Sputum induction is an easy method of obtaining samples for laboratory testing than invasive techniques like bronchoalveolar lavage. In addition, nitrate metabolites are direct markers of airway inflammation and its measurement is cheaper as compared to other markers like a fraction of exhaled nitric oxide measurement. Only, a few studies have been conducted until date to evaluate the role of sputum nitrite in measuring airway inflammation and monitoring of asthma treatment. Therefore, the current study is a landmark Indian study with larger sample size evaluating the role of sputum nitrite in the monitoring of asthmatic children.
Strengths of our study
-
Sample size is more than all the previous conducted studies
-
Applicable to general population as the method used are simple, noninvasive and cost-effective for monitoring of control of asthma
-
First Indian study which studies the correlation between induced sputum nitrite levels with clinical asthma parameters.
Limitations of the study
Controls were not included in our study.
CONCLUSIONS
This study confirms that nitrites in induced sputum correlate well with clinical asthma parameters in children with mild and moderate persistent asthma and also with asthma severity. It is a simple, noninvasive and cheap method which can be used as a parameter for monitoring of asthma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Prevalence of bronchial asthma in Indian children. Indian J Community Med. 2009;34:310-6.
- [Google Scholar]
- Prevalence of bronchial asthma in schoolchildren in Delhi. J Asthma. 1998;35:291-6.
- [Google Scholar]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention Updated. 2011. Available from: www.ginasthma.org
- [Google Scholar]
- Outgrown asthma does not mean no airways inflammation. Eur Respir J. 2002;19:284-7.
- [Google Scholar]
- Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163:1338-43.
- [Google Scholar]
- Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001;164:2107-13.
- [Google Scholar]
- Bronchoalveolar cells in children<3 years old with severe recurrent wheezing. Chest. 2002;122:791-7.
- [Google Scholar]
- Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol. 2005;16:43-51.
- [Google Scholar]
- Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722-7.
- [Google Scholar]
- Indices of airway inflammation in induced sputum: Reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 Pt 1):308-17.
- [Google Scholar]
- Cellular characteristics of sputum from patients with asthma and chronic bronchitis. Thorax. 1989;44:693-9.
- [Google Scholar]
- Measuring airway inflammation in asthma: Eosinophils and eosinophilic cationic protein in induced sputum compared with peripheral blood. J Allergy Clin Immunol. 1997;99:539-44.
- [Google Scholar]
- Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet. 2002;360:1715-21.
- [Google Scholar]
- The validity of induced sputum and bronchoalveolar lavage in childhood asthma. J Asthma. 2009;46:105-12.
- [Google Scholar]
- Exhaled nitric oxide as a marker of airway inflammation for an epidemiologic study in schoolchildren. J Allergy Clin Immunol. 2004;114:512-6.
- [Google Scholar]
- An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602-15.
- [Google Scholar]
- Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133-5.
- [Google Scholar]
- Exhaled nitric oxide in paediatric asthma and cystic fibrosis. Arch Dis Child. 1996;75:323-6.
- [Google Scholar]
- Exhaled nitric oxide measurements in normal and asthmatic children. Pediatr Pulmonol. 1997;24:312-8.
- [Google Scholar]
- Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: Effect of inhaled glucocorticoid. FASEB J. 1998;12:929-37.
- [Google Scholar]
- Nitrites in induced sputum as a simple and cheap non-invasive marker of airway inflammation for asthmatic schoolchildren. Pediatr Allergy Immunol. 2008;19:433-7.
- [Google Scholar]
- The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149(2 Pt 1):538-51.
- [Google Scholar]
- Correlation between nitrites in induced sputum and asthma symptoms in asthmatic schoolchildren. Pediatr Pulmonol. 2014;49:214-20.
- [Google Scholar]
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2006. Available from: http://www.ginasthma.org
- [Google Scholar]
- Nitric oxide metabolites in induced sputum: A noninvasive marker of airway inflammation in asthma. Indian Pediatr. 2005;42:329-37.
- [Google Scholar]
- Increased levels of nitric oxide metabolites in induced sputum and serum correlates with severity of bronchial asthma. Res J Pharm Biol Chem Sci. 2011;02:830-6.
- [Google Scholar]
- Classifying asthma severity in children: Mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426-32.
- [Google Scholar]
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma. (GINA) 2015. Available from: http://www.ginasthma.org
- [Google Scholar]
- Increased exhaled nitric oxide in asthma is mainly derived from the lower respiratory tract. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1773-80.
- [Google Scholar]
- Increased levels of nitric oxide derivatives in induced sputum in patients with asthma. J Allergy Clin Immunol. 1997;99:624-9.
- [Google Scholar]
- Nitric oxide metabolites in induced sputum: A marker of airway inflammation in asthmatic subjects. Clin Exp Allergy. 1999;29:1136-42.
- [Google Scholar]





