Translate this page into:
Intraoperative Squash Cytologic Features of Subependymal Giant Cell Astrocytoma
Address for correspondence: Dr. Jitendra Nasit, E-mail: eagleeyenasit@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Subependymal giant cell astrocytoma (SEGA) is a low grade (WHO Grade I) tumor, usually seen in patients with tuberous sclerosis complex and commonly occurs at a lateral ventricular location. Intraoperative squash cytologic features can help in differentiating SEGA from gemistocytic astrocytoma (GA), giant cell glioblastoma and ependymoma, in proper clinical context and radiological findings, which may alter the surgical management. Here, we present a case of SEGA with squash cytologic findings and a review of cytology findings of SEGA presently available in the literature. Loose cohesive clusters of large polygonal cells containing an eccentric nucleus, evenly distributed granular chromatin, distinct to prominent nucleoli, and moderate to the abundant eosinophilic cytoplasm in a hair-like fibrillar background are the key cytologic features of SEGA. Other important features are moderate anisonucleosis and frequent binucleation and multinucleation. The absence of mitoses, necrosis, and vascular endothelial proliferation are important negative features. Other consistent features are cellular smears, few dispersed cells, few spindly strap-like cells, rare intranuclear cytoplasmic inclusion, and perivascular pseudorosettes.
Keywords
Squash cytology
subependymal giant cell astrocytoma
tuberous sclerosis
INTRODUCTION
Squash cytology is now well established as an intraoperative diagnostic technique for brain tumors and also an important adjunct to the frozen section.[1234] Cytologic smears are better to visualize the fine nuclear and cytoplasmic details, which may be difficult to appreciate on frozen sections due to freezing artefacts.[125] The extent of surgery depends on squash cytologic diagnosis.[1234] Cytologic features of subependymal giant cell astrocytoma (SEGA) have not been described sufficiently due to the relative rarity of the tumor. To the best of our literature search, we found only six literature describing the cytologic findings of SEGA in 19 cases.[123456] Intraoperative diagnosis of SEGA is important, particularly if other clinical signs/symptoms of tuberous sclerosis complex (TSC) are absent or overlooked.[123456] Here we describe the squash cytologic finding of SAGE along with a relevant review of literature and discuss the differential diagnosis.
CASE REPORT
A 12-year-old-male presented with a headache, generalized tonic-clonic seizures, and weakness. Physical examination revealed few pigmented spots on the forehead and reddish spots on cheek and nose. Brain magnetic resonance imaging, revealed a 3.5 cm × 3 cm × 3 cm sized well-defined abnormal signal intensity lesion in the frontal horn of left lateral ventricle with mass effect. The mass lesion appeared hypointense in the T1-weighted image with homogenous postcontrast enhancement. Possibilities of central neurocytoma, ependymoma, and SEGA in a TSC have been raised. The patient was operated to relieve pressure symptoms of the tumor.
The specimen was sent for intraoperative squash cytology. Hematoxylin and eosin stained cytology smears showed predominantly loosely cohesive clusters of tumor cells, arranged around capillary vessels and few dispersed cells in a faint thin hair-like fibrillar background in the direction of smear. Large pleomorphic tumor cells showed large round to elongated eccentrically located nuclei, even granular chromatin, occasional distinct nucleoli, and moderate to the abundant well-defined granular eosinophilic cytoplasm, mimicking ganglionic/gemistocytic appearance. Occasional tumor cells showed parallel located nucleus. Few scattered spindly cells showed elongated nucleus looks like strap cells. Few tumor cells were giant bizarre in appearance but with even nuclear chromatin. Few binucleation and multinucleation in tumor cells were seen. Occasional intranuclear inclusions were seen. Mitoses, necrosis or endovascular proliferation was not evident in the smear [Figure 1]. In view of clinical details and radiological findings, diagnosis of SEGA was made.
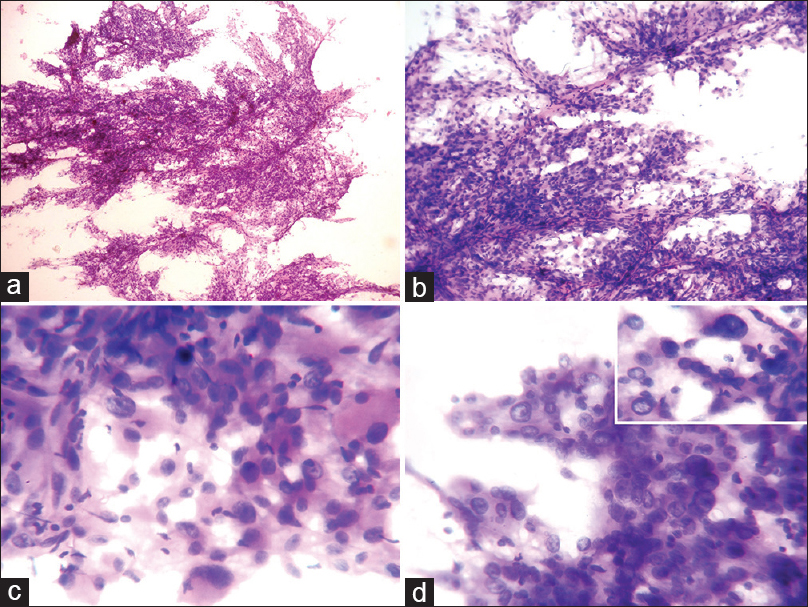
- Cytology (H and E): (a) Loose cohesive cell clusters with many thin vascular capillaries and few dispersed cells (×40). (b) Variable sized tumor cells aligned to the vessels with the meshing of thin hair-like processes, along the direction of squash smear (×100). (c) Pleomorphic tumor cells show eccentric large atypical nuclei, fine granular chromatin, occasional distinct nucleoli and abundant granular eosinophilic cytoplasm (×400). (d) Few binucleation and multinucleation in tumor cells are seen. Inset figure highlights a giant bizarre tumor cells and intranuclear inclusion (×400)
Subsequent histopathological examination of the remaining tissue showed predominantly sheets of large polygonal cells with moderate to the abundant well-defined cytoplasm with many variable sized blood vessels in a fibrillar background. The nucleus of the cell showed even granular chromatin and occasional prominent nucleoli. Frequent giant bizarre cells, binucleation, and multinucleation in tumor cells are seen. Mitoses, necrosis or endovascular proliferation was not evident [Figure 2]. Histologic findings confirm the diagnosis of SEGA.
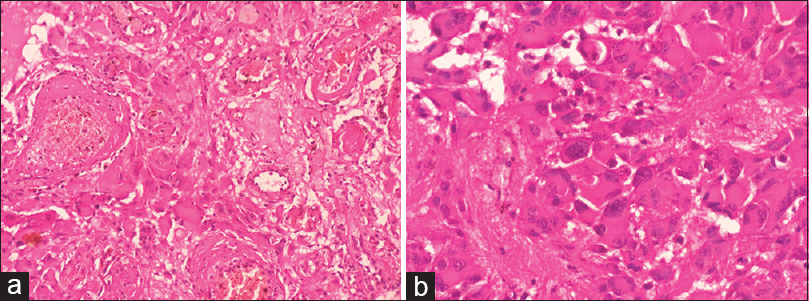
- Histology (H and E): (a) Large tumor cells are closely intermingled with variable sized blood vessels (×200). (b) Tumor cells are large, round to polygonal, displaying eccentric pleomorphic nuclei, fine granular chromatin, occasional prominent nucleoli and abundant eosinophilic cytoplasm with a well-defined border. Binucleation and multinucleation in tumor cells with fibrillary areas are seen (×400)
DISCUSSION
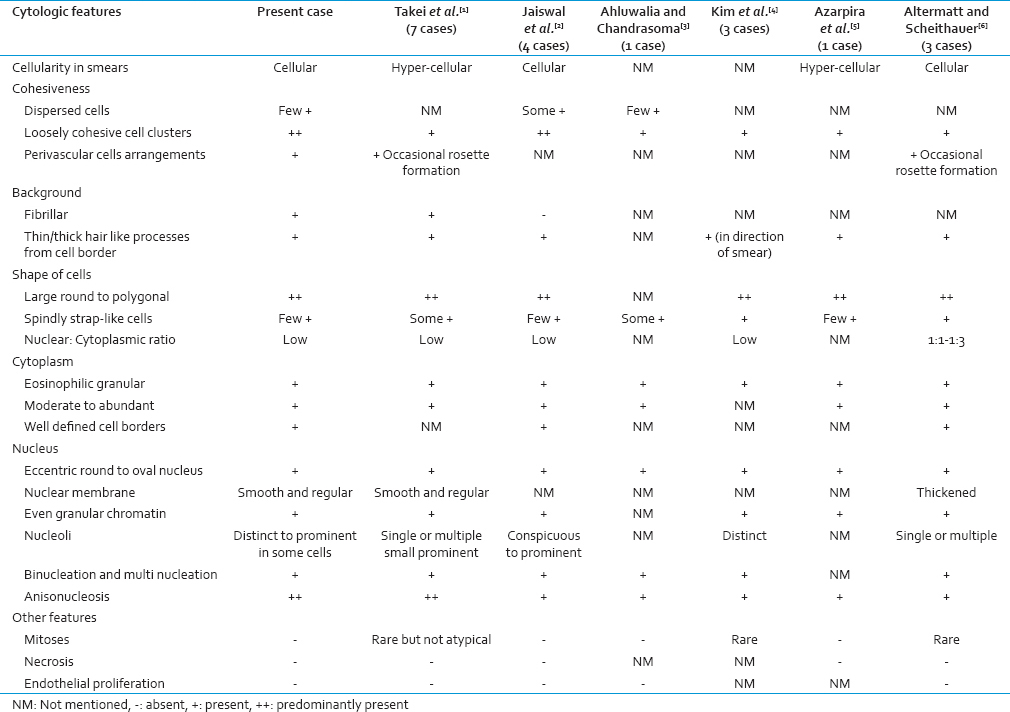
SEGA is a slow growing WHO Grade I tumor; that is classically seen in TSC.[24578] SEGA mostly occurs in the first two decades of life, with a slight male preponderance.[1234567910] It typically arises from the medial portion of lateral ventricle near the foramen of Monro. Rarely, the tumor may arise from the third ventricle.[234568] Patients commonly present with seizures and symptoms of raised intracranial pressure such as a headache, vomiting, and visual disturbances.[1567891011] Though SEGA is typical of TSC, sometimes it may be initially presenting neoplasm or often clinical findings may be overlooked or even SEGA may occur in the absence of TSC.[16812] In such instances, pathologist needs to be aware of the cytologic features of SEGA and its differential diagnosis. SEGA is generally curative with complete resection.[78911] Cytologic findings of the present case and its comparison with previously reported cases have been presented in Table 1.[123456] Loose cohesive clusters of large polygonal cells containing an eccentric nucleus, distinct to prominent nucleoli and moderate to abundant eosinophilic cytoplasm in a thin/thick hair-like fibrillar background are the key features of SEGA. Other important features are moderate anisonucleosis but evenly distributed granular nuclear chromatin and frequent binucleation and multinucleation. The absence of mitoses, necrosis, and vascular endothelial proliferation are important negative features. Other consistent features are cellular smears, few dispersed cells, few spindly strap-like cells, rare intranuclear cytoplasmic inclusion, and perivascular pseudorosettes.[123456]
SEGA should be differentiated from GA, giant cell glioblastoma, and ependymoma on cytologic ground.[1245] GA usually occurs in the brain parenchyma in contrast to the intraventricular location of SEGA. Common age for presentation of GA is the fourth decade rather than a first or second decade of SEGA. GA shows nuclear atypia, mitoses, circumferential hair-like processes (rather than straps), vertical arrangement of the nucleus in relation to the cytoplasm and absence of cohesiveness. The tumor also lacks striking cell to cell variability and multinucleation of SEGA.[23456] Giant cell glioblastoma can be reliably differentiated from SEGA based on its cytological features such as lack of cohesiveness, pronounced nuclear pleomorphism, atypia, mitoses, necrosis, and vascular endothelial proliferation.[146] Ependymoma may mimics SEGA based on clinical details like patient's age ( first or second decade), intraventricular location, and cytologically by generating cohesive clusters of tumor cells. However, cytologic features of ependymal cells are completely different from SEGA. Ependymoma shows small sized tumor cells with the absence of multinucleation and characteristic true/pseudorosette with the eosinophilic fibrillar area in between capillary lumen and neoplastic cells.[16] Rarely, SEGA can show worrisome/anaplastic features such as necrosis, microvascular proliferation, and high mitotic activity, which may mimic malignant gliomas and better termed as atypical SEGA.[781011] Atypical SEGA should not equate with aggressive behavior.[10] However, some atypical SEGA can show more aggressive behavior and requires longer follow-up.[7811] Cytologic features of atypical SEGA are not described in the literature the till date. In our opinion, smear cytology probably unable to distinguish atypical SEGA from malignant gliomas. Rare SEGA may show recurrence after surgical excision.[10] Occasional recurrence composed of predominantly blood vessels.[9] Rare cases may show intracerebral and spinal cord metastasis.[11] On immunohistochemistry, SEGA shows dual positivity for a neuronal and astrocytic markers such as synaptophysin, S-100, neurofilament, neuron-specific enolase, and glial fibrillary acidic protein.[81013] In spite of this, in the recently revised WHO classification of the central nervous system tumors, SEGA has been still included in the group of astrocytomas.[13] High frequency of BRAFV600E in SEGAs may be useful in the differential diagnosis, and also offers a potential specific treatment targeting BRAFV600E.[14] Thyroid transcription factor-1 and epithelial membrane antigen expression are seen in SEGA.[15] Ki-67 index is usually low (ranged from 0% to 8%). P53 immunoreactivity in nucleus ranged from 1% to 7.3%.[810] Testing for the mutation of TSC1 and TSC2 genes may be helpful when a patient does not fulfill criteria for the definite diagnosis.[8]
CONCLUSION
SEGA can be reliably differentiated from its close mimickers based on cytology, even when clinical findings of TSC are not present or just subtle. Loose cohesive clusters of large polygonal cells containing an eccentric nucleus, distinct to prominent nucleoli and moderate to the abundant eosinophilic cytoplasm in a hair-like fibrillar background are the key features of SEGA. Other important features are moderate anisonucleosis but evenly distributed granular nuclear chromatin and frequent binucleation and multinucleation. The absence of mitoses, necrosis, and vascular endothelial proliferation are important negative features. Other consistent features are cellular smears, few dispersed cells, few spindly strap-like cells, rare intranuclear cytoplasmic inclusion, and perivascular pseudorosettes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are thankful to Dr Amey Patankar, Head, Neurosurgical Department of Medical College and Sir Sayajirao General Hospital, Baroda, Gujarat, India.
REFERENCES
- Cytologic features of subependymal giant cell astrocytom: A review of 7 cases. Acta Cytol. 2008;52:445-50.
- [Google Scholar]
- Squash cytology of subependymal giant cell astrocytoma: Report of four cases with brief review of literature. Diagn Cytopathol. 2012;40:333-6.
- [Google Scholar]
- Cytomorphology of subependymal giant cell astrocytoma. A case report. Acta Cytol. 1993;37:197-200.
- [Google Scholar]
- Cytologic characteristics of subependymal giant cell astrocytoma in squash smears: Morphometric comparisons with gemistocytic astrocytoma and giant cell glioblastoma. Acta Cytol. 2007;51:375-9.
- [Google Scholar]
- Subependymal giant cell astrocytomas with atypical histological features mimicking malignant gliomas. Folia Neuropathol. 2011;49:39-46.
- [Google Scholar]
- Subependymal giant cell astrocytoma with atypical clinical and pathological features: A diagnostic pitfall. Cesk Patol. 2013;49:76-9.
- [Google Scholar]
- Subependymal giant cell astrocytoma associated with tuberous sclerosis exhibiting rapid regrowth after 17 years: A case report. No Shinkei Geka. 1999;27:550-6.
- [Google Scholar]
- Subependymal giant cell astrocytoma: A clinicopathological study of 23 cases with special emphasis on proliferative markers and expression of p53 and retinoblastoma gene proteins. Pathology. 2004;36:139-44.
- [Google Scholar]
- Subependymal giant cell astrocytoma with cranial and spinal metastases in a patient with tuberous sclerosis. Case report. J Neurosurg. 2004;100:498-500.
- [Google Scholar]
- Solitary subependymal giant cell astrocytoma incidentally found at autopsy in an elderly woman without tuberous sclerosis complex. Neuropathology. 2009;29:181-6.
- [Google Scholar]
- Subependymal giant cell astrocytoma (SEGA): Is it an astrocytoma. Morphological, immunohistochemical and ultrastructural study? Neuropathology. 2009;29:25-30.
- [Google Scholar]
- BRAF V600E mutations are frequent in dysembryoplastic neuroepithelial tumors and subependymal giant cell astrocytomas. J Surg Oncol. 2015;111:359-64.
- [Google Scholar]
- Thyroid transcription factor-1 and epithelial membrane antigen expression in four cases of subependymal giant cell astrocytoma. Histopathology. 2015;66:1035-6.
- [Google Scholar]





