Translate this page into:
Is There a Tendency for Thrombosis in Gestational Diabetes Mellitus?
Address for correspondence: Dr. Suheyla Gorar, E-mail: sgorar@hotmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Impact of gestational diabetes mellitus (GDM) on the coagulation system, dynamics involved at a pathophysiological level and the exact mechanism remain unclear.
Aims:
To evaluate the association between diabetes-related parameters and hemostatic factors to search for a tendency of thrombosis in GDM.
Settings and Design:
Nineteen pregnant women who had GDM, 16 healthy pregnant and 13 healthy nonpregnant controls admitted to the Endocrinology outpatient clinics were enrolled in the study.
Subjects and Methods:
Fasting and postprandial glucose, hemoglobin A1c and insulin levels, and insulin resistance; fructosamine, thrombin activatable fibrinolysis inhibitor (TAFI), tissue factor pathway inhibitor (TFPI), plasminogen activator inhibitor Type-1 (PAI-1), tissue-type plasminogen activator (t-PA), fibrinogen, plasminogen and hemoglobin levels, platelet counts, prothrombin time (PT), and activated partial thromboplastin time (aPTT) were studied.
Statistical Analysis Used:
One-way analysis of variance, Kruskal–Wallis, and post hoc Tukey honestly significant difference or Conover's nonparametric multiple comparison tests for comparison of the study groups.
Results:
PT and aPTT were significantly lower in GDM patients compared to controls (P < 0.05), whereas fibrinogen and plasminogen levels were significantly higher in this group compared to both nonpregnant and healthy pregnant controls (P < 0.05 for each). TAFI, TFPI, PAI-1, and tissue t-PA levels were not significantly different among groups.
Conclusions:
Our findings indicate tendency to develop thrombosis in GDM similar to diabetes mellitus; but more comprehensive studies with larger sample size are needed to determine the relationship between GDM and hemostasis.
Keywords
Gestational diabetes mellitus
hemostatic factors
thrombosis
INTRODUCTION
Gestational diabetes mellitus (GDM) is a frequently encountered condition during pregnancy which is related to insulin resistance and inadequate insulin secretion in response to hyperglycemia. GDM affects 2–10% of women during their pregnancy. It is important to recognize and monitor GDM closely due to the risk of adverse effects on the mother and the fetus such as development of preeclampsia, necessity for cesarean section and its associated risk of birth injuries.[1]
Impact of diabetes mellitus (DM) on the coagulation system and endothelial functions is known for many years. Hemostatic factors and activities are influenced both by the hyperglycemic state in DM and hypoglycemia induced by hypoglycemic agents. According to the medical literature, there is an increased prothrombotic state due to increased activation of platelets and prothrombotic coagulation factors coupled with a decrease in fibrinolysis.[2] Pregnancy creates a hypercoagulable state as a physiological and adaptive mechanism to ensure the hemostatic balance by preventing excessive maternal blood loss at delivery.[3] However, this physiological mechanism may convert into a pathologic process in a pregnancy complicated by GDM and/or eclampsia/preeclampsia. Since the coagulation cascade and the fibrinolytic system involve various coagulation factors interacting through complex pathways, it becomes difficult to reveal and even understand the underlying mechanisms of the hemostatic changes occurring in the glucose metabolism. Considering the impact of GDM on the coagulation system, the dynamics involved at a pathophysiological level and the exact mechanism remain still unclear.
The aim of this study was to evaluate the association between diabetes-related parameters and hemostatic factors such as thrombin activatable fibrinolysis inhibitor (TAFI), tissue factor (TF) pathway inhibitor (TFPI), plasminogen activator inhibitor Type-1 (PAI-1), tissue-type plasminogen activator (t-PA), fibrinogen, and plasminogen in GDM, thus, providing informative data about the relationship between GDM and the hemostatic system.
SUBJECTS AND METHODS
The study was conducted at the Department of Endocrinology and Metabolism of Ankara Training and Research Hospital, Ankara, Turkey, upon approval by the local Ethics Committee. Informed consent was obtained from all participants. A total of 48 women participated in the study: 19 pregnant women with a new diagnosis or untreated GDM, 16 pregnant subjects without GDM, and 13 age-matched nonpregnant healthy controls who presented to the endocrinology policlinics.
Subject selection
All pregnant women were screened for GDM between the 20th and 24th gestational week. The diagnosis of GDM was established by a 2-step oral glucose tolerance test (OGTT). The first step was a screening test. Pregnant women were given 50 g glucose orally followed by a measurement of plasma glucose level 1 h later. No restrictions were applied to the diet of the subjects. If blood glucose level was ≥140 mg/dL, a 100 g OGTT was carried out; if it was <140 mg/dL, a second test was not needed. The diagnosis of GDM was made when at least two of the following criteria were met, i.e., fasting blood glucose (FBG) ≥95 mg/dL, blood glucose ≥180 mg/dL at 1 h, ≥155 mg/dL at 2 h, ≥140 mg/dL at 3 h of the OGGT. Exclusion criteria for the study were history of DM or GDM during former pregnancies, liver dysfunction (any prior diagnoses of cirrhosis, hepatitis, and/or known liver function test abnormalities), renal dysfunction (any prior diagnoses of acute kidney injury and/or chronic kidney disease), preeclampsia/eclampsia, and endocrine disorders (acromegaly, Cushing disease, and thyroid dysfunction), use of medications with known effects on glucose metabolism, and hemostatic system such as antidiabetic medications, aspirin, heparin, and enoxaparin.
Study procedures
All participants underwent a comprehensive physical examination. Body mass index (BMI) was calculated (BMI = body weight [kg]/square of height [m2]). Venous blood samples were collected from all subjects in the morning after an overnight fasting. FBG, postprandial blood glucose (PPBG), blood insulin, fructosamine, hemoglobin A1c (HbA1c) and hemoglobin levels, platelet counts, prothrombin time (PT), activated partial thromboplastin time (aPTT), plasma fibrinogen, and plasminogen levels were measured. Insulin resistance was calculated for each patient using the homeostasis model assessment insulin resistance index (HOMA-IR) by the formula of “FBG (mmol/L) × fasting insulin (mIU/mL)/22.5”.
Laboratory tests
The coagulation tests were performed via calibrated kits used in routine laboratory practice. For specific coagulation tests including TAFI, TFPI, PAI-1, and t-PA, a total of 4 mL of blood sample was collected into standard tubes containing 0.5 mL of 0.109 M trisodium citrate. Platelet-poor plasma was obtained by centrifugation at 3500 g at 10°C for 20 min. The plasma sample was stored at −80°C until it was analyzed. All tests were carried out by enzyme-linked immunosorbent assay using commercial kits (American Diagnostica, Stamford, the USA). According to the manufacturer's instruction, normal ranges were 40.0–250.0% for TAFI; 75.0–120.0 ng/mL for TFPI; 4.0–43.0 ng/mL for PAI-1; and 0–9 ng/mL for t-PA.
All laboratory studies were conducted at the Haematology Laboratories of the Ankara Training and Research Hospital, Ankara, Turkey.
Statistics
Statistical analysis was performed by using SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL, USA). The distribution pattern of continuous variables was determined by Shapiro-Wilk test; and homogeneity of variances was evaluated by Levene test. Data were expressed as mean ± standard deviation (SD) or median (interquartile range), where applicable.
The differences in means among groups were analyzed using one-way ANOVA while Kruskal–Wallis test was used for the comparison of medians. When the P value showed statistical significance, post hoc Tukey honestly significant difference, or Conover's nonparametric multiple comparison tests were used to test the significance of differences between pairs of results. A P < 0.05 was considered statistically significant.
RESULTS
The mean age SD of the GDM patients, and healthy pregnant and nonpregnant control groups were 31.2 ± 6.0 years, 26.8 ± 5.0 years, and 32.0 ± 8.7 years, respectively. The main characteristics of the study population are summarized in Table 1. There were no differences between the groups in regards to the mean age, FBG and insulin levels, and HOMA-IR. Compared with controls, BMI was significantly higher in GDM patients (P < 0.01). There was no statistically significant difference between pregnant women with and without GDM in terms of mean BMI. PPBG levels in GDM patients were significantly higher than both pregnant and nonpregnant healthy control groups (P < 0.01, for each). In the pregnant group without GDM, HbA1c and fructosamine levels were lower compared to the control and GDM groups (P < 0.05, P < 0.01; respectively).
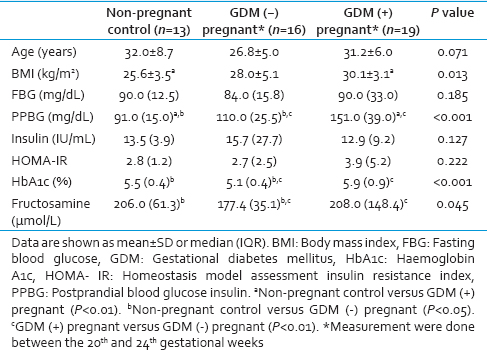
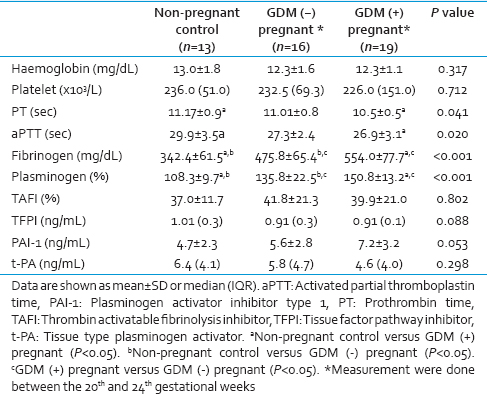
Hemoglobin levels and platelet counts were not significantly different between the study groups [Table 2]. PT and aPTT were significantly lower in GDM patients compared to the controls (P < 0.05). Fibrinogen and plasminogen levels were significantly higher in the GDM group compared to the healthy pregnant and nonpregnant control groups (P < 0.05, for each). TAFI, TFPI, PAI-1, and t-PA levels were not found to be significantly different between groups. Although no statistically significant difference was demonstrated between the groups, average PAI-1 level was remarkably higher while t-PA level tended to be lower in GDM patients compared the other study groups.
DISCUSSION
The hemostatic system involves an excellent balance. Production and activation of prothrombotic and fibrinolytic factors are managed very well under normal circumstances. However, this balance is shifted toward a prothrombotic state and hypofibrinolysis in patients with DM. Plasma levels of clotting factors such as fibrinogen; factors (F) VII, VIII, XI, and XII; PAI-1; and von Willebrand factor increase while fibrinolysis is inhibited due to the compact structure and innate resistance of the fibrin molecule to lysis in DM.[4] Nevertheless, present studies are not able to explain the final impact of DM and a hyperglycemic state on hemostasis, including the complex interactions and pathophysiological mechanisms it involves.
GDM may actually be presenting similar to a milder form of the metabolic syndrome. Since BMI is higher in women with GDM compared to healthy subjects; insulin resistance may develop in pregnant subjects leading to GDM. Laboratory findings indicating insulin resistance and DM such as elevated FBG and/or PPBG, fasting insulin, and HbA1c levels and HOMA-IR have been demonstrated in GDM. Our data revealed that BMI, PPBG, HbA1c, and fructosamine levels were higher in GDM patients compared to healthy pregnant and nonpregnant control subjects, being in accordance with the medical literature. Based on this information, it can be presumed that in GDM, a hypercoagulable state may ensue following the changes in the level of hemostatic factors, similar to DM.
Fibrinogen is a complex glycoprotein synthesized by hepatocytes. While it is primarily involved in fibrin clot formation, platelet aggregation, and wound healing, its level rises in response to inflammation or tissue injury as an acute phase reactant. The balance between fibrin clot formation and fibrinolysis determines whether the clinical manifestations of abnormal fibrinogen levels include bleeding, thrombosis, both, or neither. As shown in the medical literature, fibrinogen increases not only in a normal pregnancy but also in pregnancy-related complications such as GDM, hypertension, and preeclampsia. It was reported that BMI and fibrinogen levels in GDM patients were higher compared to pregnant subjects without GDM.[5] It was suggested that the only indicator of a tendency toward hypercoagulability was the fibrinogen level in GDM. In another study, high fibrinogen levels were found in patients with GDM as compared to both nonpregnant and healthy pregnant women.[6] They reported a higher incidence of thrombophilia in women with GDM compared to healthy pregnant subjects. Similarly, we also showed that fibrinogen levels were higher in GDM patients and healthy pregnant subjects compared to nonpregnant controls in this study.
Plasminogen is a glycoprotein which can be converted to plasmin, a serine protease that degrades fibrin and causes lysis of thrombus by plasminogen activators such as t-PA and urinary-t-PA (u-PA, urokinase). t-PA is secreted by vascular endothelial cells and circulates in plasma in trace concentrations. It is primarily responsible for catalyzing the degradation of intravascular fibrin by converting plasminogen to plasmin. However, u-PA is secreted by a variety of cell types, including nonvascular cells. Besides activation of plasminogen to plasmin, it can also carry out degradation or activation of variety of proteins that have key roles in cell function and migration. PAI-1 is the primary inhibitor of both t-PA and u-PA. By this action, it suppresses plasmin formation and fibrinolysis. It is secreted by multiple cell types, including vascular endothelium and smooth muscle cells.[7]
Our knowledge on changes of these coagulation factors is very limited and controversial in GDM. It was shown in a study that t-PA level was increased, and PAI was unchanged in GDM[8], whereas in another study, t-PA was found to be lower and PAI obviously higher in GDM patients.[9] On the other hand, PAI-1 levels were not found to be different between pregnant women with normal glucose tolerance and GDM patients.[10] We found in this study that t-PA and PAI-1 levels were not significant between groups, but t-PA was the highest in the nonpregnant control group, and the lowest in GDM group while PAI-1 was higher in the GDM group compared with the other two groups. Furthermore, plasminogen which is known to be a prothrombotic factor was found remarkably increased in GDM.[911] In our study, plasminogen level was the highest among the GDM patients and also significantly higher in the pregnant group without GDM than the nonpregnant control group.
TAFI is a procarboxypeptidase that is activated by the thrombin-thrombomodulin complex and cleaves C-terminal lysine residues from fibrin fragments (to which plasminogen binds with high affinity), thereby disrupting recruitment of plasminogen to the partially digested fibrin clot, inhibiting fibrinolysis.[12] TFPI circulates in plasma and inhibits factor-X activation in two ways. First, it directly inhibits Factor Xa (FXa); and second, it complexes with FXa, and this complex inhibits TF/FVIIa, thereby impairing the triggering mechanism of the extrinsic pathway. The consequence of this is the reduced fibrin clot formation. It is primarily synthesized by the microvascular endothelium.[13] PAI-1 and TAFI levels in GDM were investigated and high PAI-1 levels were found to be associated with GDM, whereas TAFI increased in only pregnant women, while there was no statistically significant difference between GDM and pregnant controls.[14] On the other hand, it was reported that TAFI was high in GDM and suggested that it may have a role in increased risk of hypercoagulability.[15] TAFI activity was shown to increase in pregnancy compared to nonpregnant women up to different levels in different stages of pregnancy and after delivery.[16]
To the best of our knowledge, no studies were found in the medical literature about GDM and TFPI relationship. However, there are also many various, ambiguous and often conflicting studies about TFPI in women with normal and complicated pregnancy, especially with eclampsia/preeclampsia.[17] In a study investigated plasma TF and TFPI levels in pregnancies with preeclampsia, the researchers have determined that TFPI was lower in preeclampsia, and TF may contribute the pathological hypercoagulable state in this condition.[18] On the contrary, another study indicated significantly high TFPI plasma levels in severe preeclampsia and TF did not vary in this state.[19] GDM which is a complication of pregnancy such as eclampsia/preeclampsia, neither TAFI nor TFPI was found to be significantly different between pregnant women with and without GDM and also nonpregnant healthy control in our study.
The most important measures in assessing the coagulation cascade are PT and aPTT. There are studies showing that decreased PT and aPTT support hypercoagulability status in both normal pregnancy and GDM. It was determined that aPTT and platelet counts were significantly lower in the 3rd trimester of normal pregnancy.[20] However, another group reported that both parameters were unchanged in women with GDM.[11] In our findings, PT and aPTT were lower in GDM group than nonpregnant controls while both parameters were not significantly different in healthy pregnant subjects than nonpregnant controls and those with GDM. We think that this data support hypercoagulable state in GDM.
The sample small size was the most important limitation of our study, which might have prevented the detection of significant correlations and/or differences among the groups. Second, other hemostatic factors should have been included, which might have provided evidence of relationship between GDM and hemostasis. Third, these factors should have also been measured after delivery. Despite these limitations, we hope that our data will enlighten this topic to some extent.
CONCLUSION
There is general opinion based on encouraging data that GDM, which complicates pregnancy by exposing prothrombosis and hypofibrinolysis that may be dangerous both for the mother and the baby. We believe that levels of coagulation factors may vary in different stages of pregnancy and postpartum period with diverse etiopathogenesis. Similar to previously reported studies, our study suggests that GDM may play a role in the pathogenesis leading to a thrombotic tendency similar to DM. Further clinical studies at larger scales are needed to further delineate the relationship between GDM and homeostasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-43.
- [Google Scholar]
- Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015;70(Suppl 1):78-86.
- [Google Scholar]
- Haemostatic and cytokine changes in gestational diabetes mellitus. Gynecol Endocrinol. 2011;27:356-60.
- [Google Scholar]
- Changes in hemostasis and fibrinolysis in gestational diabetes. Cas Lek Cesk. 1996;135:106-10.
- [Google Scholar]
- PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157:291-8.
- [Google Scholar]
- Coagulation and fibrinolysis parameters in normal pregnancy and in gestational diabetes. Am J Perinatol. 1998;15:479-86.
- [Google Scholar]
- Value of coagulation function and fibrinolytic system assessment in patients with gestational diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:35-7.
- [Google Scholar]
- Fibrinolytic dysfunction after gestation is associated to components of insulin resistance and early type 2 diabetes in latino women with previous gestational diabetes. Diabetes Res Clin Pract. 2007;78:340-8.
- [Google Scholar]
- Haemostatic changes in gestational diabetes mellitus. Int J Diabetes Dev Ctries. 2015;35(Suppl 3):S502-6.
- [Google Scholar]
- Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U) J Thromb Haemost. 2003;1:1566-74.
- [Google Scholar]
- Kinetics of the inhibition of factor Xa and the tissue factor-factor VIIa complex by the tissue factor pathway inhibitor in the presence and absence of heparin. Biochemistry. 1994;33:12686-94.
- [Google Scholar]
- Gestational diabetes has no additional effect on plasma thrombin-activatable fibrinolysis inhibitor antigen levels beyond pregnancy. Diabetes Res Clin Pract. 2008;81:93-6.
- [Google Scholar]
- Levels of thrombin activatable fibrinolysis inhibitor in gestational diabetes mellitus. Gynecol Endocrinol. 2013;29:327-30.
- [Google Scholar]
- Changes in activity of plasma thrombin activatable fibrinolysis inhibitor in pregnancy. Gynecol Obstet Invest. 2004;58:19-21.
- [Google Scholar]
- The role of tissue factor in normal pregnancy and in the development of preeclampsia: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:192-6.
- [Google Scholar]
- The relationship between plasma and placental tissue factor, and tissue factor pathway inhibitors in severe pre-eclampsia patients. Thromb Res. 2010;126:e41-5.
- [Google Scholar]
- Preeclampsia: The role of tissue factor and tissue factor pathway inhibitor. J Thromb Thrombolysis. 2012;34:1-6.
- [Google Scholar]
- Normal pregnancy and coagulation profile: From the first through the third trimester. Niger J Med. 2015;24:54-7.
- [Google Scholar]





