Translate this page into:
Laboratory Diagnosis of Tuberculous Meningitis - Is There a Scope for Further Improvement?
Address for correspondence: Dr. Rajeev Thakur, E-mail: drrajeevthakur@rediffmail.com
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims:
Tuberculous meningitis (TBM) still remains a diagnostic challenge because of inconsistent clinical presentation and lack of rapid, sensitive and specific tests. This study was carried out to diagnose TBM by a combination of direct microscopy on Ziehl-Neelsen (ZN) staining, culture by conventional Lowenstein Jensen (LJ) media and Bactec MGIT 960 system in clinically suspected cases, supported by laboratory parameters.
Materials and Methods:
A total of 164 cerebrospinal fluid (CSF) samples from suspected cases of TBM were processed for direct acid fast bacilli (AFB) smear examination, and culture on Bactec MGIT 960 and LJ media.
Results:
AFB were detected on direct smears in 13 of 164 (7.9%) specimens and Mycobacterium tuberculosis was isolated by at least one of the culture methods from 49 (29.8%) CSF samples, of which 45 (27.4%) were detected positive for M. tuberculosis by MGIT 960 culture and 18 (10.9%) by culture on LJ medium. The mean time of detection in MGIT and LJ media for M. tuberculosis were 18 and 38 days, respectively.
Conclusions:
A combination of laboratory parameters like smear microscopy, conventional culture and automated method like Bactec MGIT increases the sensitivity of diagnosing TBM as compared to any single method.
Keywords
Bactec MGIT 960
cerebrospinal fluid
tuberculous meningitis
INTRODUCTION
Tuberculosis (TB) remains a major global health problem with 7-8 million new cases reporting every year and the mortality ranging from 1.6 to 2.2 million per year.[1] In India, 2.2 million people are affected every year and approximately 400,000 people die due to TB.[2] Extrapulmonary tuberculosis accounts for 10-15% of all the TB cases and approximately 5-15% of these patients develop neurologic involvement.[2,3] Tuberculous meningitis (TBM) is a medical emergency and is the most malignant form of neurotuberculosis.[3] In untreated TBM, the case fatality rate is almost 100% and delay in treatment may lead to permanent neurologic damage.[3] Early diagnosis and prompt institution of anti-tuberculous treatment are the deciding factors for the final outcome of the patient.
Till date, the diagnosis of TBM remains a challenge. The sensitivity of acid fast bacilli (AFB) microscopy and conventional Lowenstein Jensen (LJ) culture is quite low, especially in cerebrospinal fluid (CSF) which contains a small number of organisms.[3,4] A variety of commercially available automated systems like Bactec MGIT 960 (Becton Dickinson, Sparks, MD, USA), radiometric Bactec 460 (Becton Dickinson, Heidelberg, Germany), MB Bact (Organon Teknika, Boxtel, The Netherlands) and ESP II (Difco Laboratories, Detroit, MI, USA) have been evaluated for the rapid detection of mycobacteria in clinical specimens.[5-7] Bactec MGIT 960 has been shown to be a safe, sensitive and less labor-intensive method as compared to other automated methods.[5-7] This system is fully automated with a high capacity, and is non-radiometric and non-invasive culture-based method. The culture tubes (MGIT) contain modified Middle Brooks 7H9 broth with a fluorescent growth indicator embedded in silicone at the bottom of each tube, which is sensitive to the presence of oxygen in the broth. The initial concentration of dissolved oxygen quenches the emission from the indicator and little fluorescence can be detected. Later, actively growing and respiring mycobacteria consume the oxygen that allows the compound to fluoresce. Fluorescence is detected by means of instrument photodetectors which correspond to the amount of oxygen consumed by mycobacteria. The instrument automatically tests the tubes continuously every 60 minutes and flags the culture as soon as they become positive. Nucleic acid amplification techniques like polymerase chain reaction (PCR) for early diagnosis of TBM have been attempted with mixed success and need further evaluation to demonstrate improvement in their diagnostic and clinical utility.[8,9]
Several studies have been done to detect Mycobacterium tuberculosis in respiratory and extrapulmonary samples but data on CSF samples are scanty.[4,10-13] Any single conventional or automated method for the diagnosis of TBM has limitations of sensitivity; so this study was carried out to diagnose TBM by a combination of conventional and automated culture methods coupled with high index of clinical and laboratory suspicion.
MATERIALS AND METHODS
Clinical specimens
This prospective study was conducted in the department of microbiology of a tertiary care hospital over a period of 2 years. A total of 164 patients (age 8-84 years), clinically suspected to be suffering from TBM with abnormal biochemical (protein > 20-1000 mg/dl, sugar <45 mg/dl) and cytologic parameters (>5 leukocytes/mm3), were processed as per standard protocol.[3,14] No patient was on antitubercular therapy at the time of presentation.
Processing
CSF aliquots of 3-5 ml were concentrated by centrifugation (3000 Χg for 10 minutes) and the sediments were used to prepare smears for India ink, direct smear examination by Ziehl-Neelsen (ZN) stain. CSF was cultured in conventional LJ media along with Bactec MGIT 960 system (Becton Dickinson Diagnostic Instrument Systems, Sparks,M.D,USA). LJ media were incubated under an atmosphere containing 5% CO2 at 37°C and were observed on a weekly interval till 8 weeks, whereas Bactec MGIT 960 cultures were processed till 6 weeks.[14]
Culture in Bactec MGIT 960 system for M. tuberculosis was done strictly according to the manufacturer's instructions. Lyophilized MGIT PANTA (containing polymyxin B, azlocillin, nalidixic acid, trimethoprim, amphotericin B) was reconstituted with MGIT growth supplement OAEDC (containing oleic acid, albumin, dextrose, catalase, polyoxyethylene stearate), and 0.8 ml of this was added prior to sample inoculation to the MGIT. Smears were made from all positive culture tubes as well as from MGIT 960 negative tubes that had some deposit in them, to confirm the presence or absence of mycobacteria. Samples positive for AFB either by LJ culture or Bactec MGIT 960 were later subjected to para-nitrobenzoic acid (PNB) test to rule out non-tuberculous mycobacteria (NTM) (PNB resistant) as per standard protocol.
RESULTS
Capsulated budding yeast cells suggestive of Cryptococcus neoformans were seen in India ink in 11 of the 164 (6.7%) CSF samples. All these CSF samples were still subjected to culture for mycobacterium (both conventional and automated) to rule out concomitant tuberculous and cryptococcal CNS infection.
On ZN staining, AFB was seen in 13 of the 164 (7.9%) samples. In LJ media, M. tuberculosis was isolated in 18 and NTM in two samples. By Bactec MGIT 960, positive signals were detected in 57 of the 164 samples. Out of these, 43 were identified as M. tuberculosis by PNB test and 5 as NTM. The remaining nine, Bactec MGIT 960 positive samples, were found to have Cryptococcus in six and other contaminants in three samples when examined microscopically (false positives). Bactec gave false negative signal in two samples which were later detected to be AFB positive by microscopy from the tube deposit. Thus, a total of 45 (27.4%) clinical specimens were positive for M. tuberculosis by Bactec MGIT [Table 1].
| MGIT + ve LJ + ve | MGIT + ve LJ - ve | MGIT - ve LJ + ve | |
|---|---|---|---|
| Smear positive (n = 13) | 12 | 1 | 0 |
| Smear negative (n = 36) | 2 | 30 | 4 |
A total of 49 (29.8%) CSF samples were culture positive by the combined use of these two culture methods. As a single medium, Bactec MGIT 960 recovered M. tuberculosis from 45 (27.4%) CSF samples and LJ medium recovered the same from 18 (10.9%) samples. Bactec MGIT was negative in 4 LJ culture positive samples and LJ medium did not reveal growth in 31 MGIT positive samples. Figure 1 depicts the diagnosis of TBM by single as well as combination of two or more methods. No case of concomitant tuberculous and cryptococcus meningitis was detected.
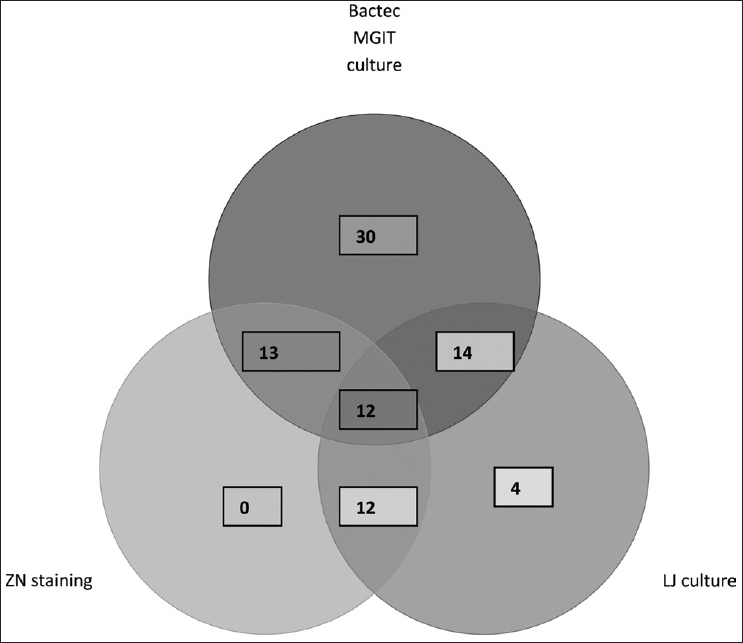
- Positivity by ZN staining, culture on LJ medium and Bactec MGIT 960 system in patients of TBM (n = 49)
The average time to identify M. tuberculosis was 18 days (11-28 days) with MGIT 960 and 38 days (28-50 days) in LJ media.
DISCUSSION
The diagnosis of TBM was made by culture in 29.8% of patients in contrast to 8.7-18.36% in previously published studies on CSF samples.[4,5,10,11] This higher positivity could have been due to strict inclusion criteria based on clinical, biochemical and cytologic parameters in this study rather than only on the basis of clinical suspicion.
In this study, smear positivity for AFB was 7.9%. Several workers have shown smear positivity for AFB in CSF by microscopy to be <10% which is in concordance with our study.[3,9] It has been recommended that collection of four serial samples and spinning of large volumes (10-20 ml) of CSF for 30 minutes could enhance the rate of detection in smear microscopy.[3] However, repeated collection of such large volume of CSF is practically not possible. Though less sensitive, smear microscopy is a simple, rapid, cost effective adjunct for the diagnosis of TBM.
In culture, the automated Bactec MGIT 960 system displayed a higher rate of recovery of M. tuberculosis (27.4%) than LJ media (10.9%). Various authors have reported detection rates ranging from 4.3 to 48.9% for MGIT 960 and from 10.2 to 55.8% for LJ medium, which is in accordance with our study.[4,10,13] Bactec MGIT system showed higher sensitivity probably because of added growth supplements like OAEDC and antibiotics like PANTA which allowed even small number of microorganisms to grow as compared to LJ. However, this system gave false negative signal in two tubes, despite the presence of mycobacterial growth in the medium. Similar findings of mycobacterium growth with a negative signal have been reported by Sankar et al.[15] Finding mycobacterium in signal negative culture is alarming and all the Bactec MGIT tubes with negative signal must be rechecked before reporting negative for M. tuberculosis. There were four culture positive samples which yielded growth only on LJ media probably due to some growth promoting factors present in egg-based media. These four positive samples would have been missed if LJ media were not incorporated along with MGIT 960. Thus, it is important that conventional solid media should always be used along with Bactec.
Besides higher isolation rate, the mean time to detect M. tuberculosis was approximately half in Bactec MGIT 960 as compared to LJ media (18 days vs. 38 days), which is consistent with other reports.[4] Some studies have reported mean time for detection to be less in smear positive cases but in this study no difference in detection time was observed in smear positive vs. negative samples.[5] Besides decreasing the time for initial isolation, Bactec has an additional advantage of earlier drug susceptibility testing than LJ medium, which has implications in monitoring treatment failure in TBM patients.
No case of tuberculous and cryptococcal meningitis coinfection was detected in our study. This was either due to true absence of dual infection or inability to continue incubation for the recommended duration due to growth of Cryptococcus within 1 week in both the culture media. Interestingly, PANTA in MGIT tubes has amphotericin B to inhibit the fungus but Cryptococcus was able to grow in 6 of the 11 Cryptococcus positive cases.
Our study demonstrates that though MGIT 960 system has a better sensitivity for diagnosis of TBM, both solid and liquid media are necessary to maximize the sensitivity of detection. The importance of smear microscopy and conventional culture cannot be undermined despite the availability of latest diagnostic techniques. It needs to be emphasized that strict clinical and laboratory inclusion criteria are needed to improve the sensitivity of detection of M. tuberculosis in TBM. A combination of methods works better than any single method and other modalities like antigen detection and molecular techniques need to be combined with smear microscopy and culture for further improvement. Though encouraging results have been shown by some of these newer techniques, they have not significantly improved upon the sensitivity of detection by culture. The studies evaluating the role of PCR for detection of M. tuberculosis have used limited number of CSF samples and have demonstrated variable sensitivity (30-90%) and specificity (95-100%).[16] Importantly, the gold standard for comparison in these studies had been culture. Therefore, well-characterized longitudinal studies employing combination of conventional, newer and molecular techniques with significant number of CSF samples would probably answer the intriguing question of further improvement in precise and specific diagnosis of TBM.
Source of Support:
Nil
Conflict of Interest:
None declared.
REFERENCES
- Mycobacterium bovis BCG based vaccine against tuberculosis: novel developments. Vaccine. 2003;21:667-70.
- [CrossRef] [PubMed] [Google Scholar]
- Training module for medical practitioners. Central TB division. Directorate General of health services, Ministry of Health and family welfare, Nirman Bhawan, New Delhi. Available from: http://www.tbcindia.org (accessed )
- [Google Scholar]
- Pathogenesis, diagnosis, treatment, and outcome aspects of cerebral tuberculosis. Med Sci Monit. 2004;10:RA215-29.
- [Google Scholar]
- Comparative evaluation of Bactec 460TB system and Lowenstein Jensen medium for the isolation of M. tuberculosis from cerebrospinal fluid samples of tuberculous meningitis patients. Ind J Med Microbiol. 2007;25:236-40.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of recoveries of Mycobacterium tuberculosis using the Automated BACTEC MGIT 960 System, the BACTEC 460 TB System, and Lφwenstein-Jensen Medium. J Clin Microbiol. 2000;38:2395-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of the Mycobacteria Growth Indicator Tube (MGIT) compared with radiometric (Bactec) and solid media for isolation of Mycobacterium species. J Med Microbiol. 1998;47:821-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the Automated Mycobacteria Growth Indicator Tube System (BACTEC 960/MGIT) with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Am J Clin Pathol. 2002;118:542-5.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev. 2004;17:903-25.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Roche AMPLICOR Mycobacterium tuberculosis PCR in early diagnosis of tuberculous meningitis. J Clin Microbiol. 1998;36:1251-4.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study for the detection of mycobacteria by bactec MGIT 960, Lowenstein Jensen media and direct AFB smear examination. Indian J Med Microbiol. 2007;25:383-6.
- [CrossRef] [PubMed] [Google Scholar]
- Use of Bactec 460 Tb system in the diagnosis of tuberculosis. Indian J Med Microbiol. 2007;25:32-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of rapid colorimetric method with conventional method in isolation of Mycobacterium tuberculosis. Indian J Med Microbiol. 2004;22:44-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the conventional diagnostic modalities, bactec culture and polymerase chain reaction test for diagnosis of tuberculosis. Indian J Med Microbiol. 2005;23:29-33.
- [CrossRef] [PubMed] [Google Scholar]
- Meningitis and other infections of Central Nervous system, chapter 58 In: Bailey and Scott′s Diagnostic Microbiology. (11th). 2002. p. :907.
- [Google Scholar]
- Recovery of Mycobacterium tuberculosis from sputum treated with Cetyl Pyridinium Chloride. J Clin Microbiol. 2009;47:4189-90.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of the polymerase chain reaction in the diagnosis of tuberculous meningitis. Res Microbiol. 2006;157:967-70.
- [CrossRef] [PubMed] [Google Scholar]





