Translate this page into:
Laboratory Evaluation of Three Regimens of Treatment of Chronic Hepatitis B: Tenofovir, Entecavir and Combination of Lamivudine and Adefovir
Address for correspondence: Prof. Sarman Singh, E-mail: sarman_singh@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Chronic hepatitis B is a disease of concern due to its life-threatening complications like cirrhosis, and hepatocellular carcinoma (HCC) in 20-40% of patients. There are about 400 million people affected worldwide with HBV, and over 300,000 die every year from HBV-related diseases. Oral antivirals like lamivudine, adefovir, entecavir, and tenofovir are commonly used to treat chronic hepatitis B. In this study, we tried to evaluate the comparative efficacy of these drugs alone and in combination.
Materials and Methods:
Chronic hepatitis B patients with HBV-DNA more than 104 Copies/mL irrespective of their HBeAg status (n = 60) were enrolled in a prospective study. 21, 20, and 19 patients were treated with lamivudine (100 mg/day) plus adefovir (10 mg/day) combination entecavir monotherapy (0.5 mg/day) and tenofovir monotherapy (300 mg/day), respectively and were followed up for 24 weeks with their virological, serological, and biochemical markers measured at 12 and 24 weeks.
Results:
After 24 weeks of treatment, there was no significant difference between the 3 groups in suppressing HBV-DNA to undetectable levels. The median decrease in HBV-DNA levels from baseline was better with tenofovir and entecavir monotherapies than lamivudine and adefovir combination, which was statistically significant. There was no significant difference between the 3 groups in HBsAg and HBeAg seroconversion and normalization of biochemical parameters.
Conclusion:
Entecavir and tenofovir monotherapy were found to be more effective than lamivudine plus adefovir combination in reducing the HBV-DNA levels. However, lamivudine plus adefovir combination was not too inferior, especially when cost of treatment was taken into consideration.
Keywords
Adefovir
entecavir
lamivudine
tenofovir
INTRODUCTION
Chronic infection with hepatitis B virus (HBV) is a global public health problem, often called the ‘silent killer’, which eventually leads to liver cirrhosis, decompensated hepatic disease, or hepatocellular carcinoma in 20-40% of patients.[1] The virus belongs to the Family Hepadnavirida, genus Orthohepadnavirus and species Hepatitis B virus.[2] About 400 million people are affected worldwide with the HBV disease mainly from developing countries, and it is estimated that between 200,000-300,000 die every year from HBV-related cirrhosis and hepatocellular carcinoma, respectively.[3] Hence, effective and affordable antiviral therapy has become a priority research area. Most of the available oral drugs are targeted to suppress the HBV-DNA replication. Accordingly, to evaluate the efficacy of any new drug or combination of drugs, levels of HBV-DNA are measured.
Antiviral drugs commonly used in India for the treatment of chronic HBV infection include interferon α, peginterferon α2a, lamivudine, adefovir dipivoxil, entecavir, telbivudine, and tenofovir disoproxil fumarate. Since interferons are expensive and have disadvantages like parenteral administration and treatment-limiting side effects, oral nucleoside and nucleotide analogues are preferred.[4]
Lamivudine is the first nucleoside analogue approved against HBV in the year 1995 by the Food and Drug Administration (FDA), and studies show that it can suppress the HBV-DNA by 5 logs at a daily dose of 100 mg for 48-52 weeks.[5] But, the emergence of tyrosine-methionine-aspartate-aspartate (YMDD) mutation has limited its long term efficacy by conferring resistance by a substitution in the reverse transcriptase region of HBV polymerase, usually a methionine to valine or isoleucine at amino acid position 204 (rtM204I/V) in the (YMDD) motif, which is often accompanied by a compensatory substitution at position 180 (rtL180M).[6] Hence, research for finding new drugs has been a priority. Adefovir dipivoxil, an acyclic nucleotide analogue, was approved by FDA in 2002. It is given at an oral dose of 10 mg daily, which can suppress the HBV-DNA replication by 4 logs by 48 weeks and is equally effective as lamivudine in treating chronic HBV infection.[5] Studies show that adding adefovir to lamivudine prevents the emergence of lamivudine resistance and vice-versa and hence the combination is effective in suppressing HBV-DNA replication.[7–9]
A new drug, entecavir, an oral deoxyguanosine analogue, was approved by FDA in 2005. Studies show that it is more potent than other available drugs suppressing HBV-DNA to 6.9 logs at a daily dosage of 0.5 mg by 48 weeks. Since the development of resistance to entecavir requires the selection of a primary resistance mutation at codon 204, with or without the compensatory mutation rtL180M, followed by the addition of secondary resistance mutations (at codons 184, 202, or 250), it is considered as high genetic barrier drug.[1011]
A more recent drug, tenofovir, approved by FDA in 2008, is an oral nucleotide analogue. It is another potent antiviral drug, which acts against HBV. Studies show that it suppresses HBV-DNA by 5.3 logs at a daily dosage of 300 mg for 48 weeks.[12] Though cross-resistance of tenofovir with other HBV reverse transcriptase inhibitors have been observed, its clinical significance is not well-observed.[13] Tenofovir is also demonstrated to be effective in patients with lamivudine resistance and adefovir failure.[14]
Chronic hepatitis B virus infection is a major concern for many developing countries like India. About 75% HBV cases are reported from Asian countries.[15] Studies show that chronic hepatitis B is more prevalent among the low socio-economic population, most of whom could not afford to get adequate treatment due to high cost of drugs and supportive laboratory test. The investigations cost around 80-100 US$ per visit, and the follow-up has to be continued for 2 years. Thus, making the total cost to around 500-600 US$ only for monitoring the disease and drug efficacy. Adding the monthly cost of drugs becomes beyond the reach of most Indian patients, with an average daily income of just US $1.25. The monthly costs of lamivudine plus adefovir combination, entecavir or tenofovir are US $ 200, 560, and 380, respectively.
The purpose of this study was to compare the efficacy of lamivudine plus adefovir combination vs. entecavir monotherapy vs. tenofovir monotherapy in suppressing HBV-DNA. The secondary objective was also to see the normalization speed of serological and biochemical markers in chronic hepatitis B infection. The data generated through this study is expected to provide an insight in decision making to choose the best treatment option suitable for a particular economic group.
MATERIALS AND METHODS
Patient selection
This study was conducted in the All India Institute of Medical Sciences, New Delhi, India between January 2010 and February 2011. The study was approved by the institutional ethical committee. This was a prospective cohort study. Having the primary objective as reduction in the HBV-DNA to undetectable levels (<400 copies/mL), with the confidence interval of 95% and power of 90%, a sample size of 15 in each group was calculated. Chronic hepatitis B patients attending the gastroenterology outpatient department were recruited after satisfying the inclusion criteria, which included history of hepatitis B antigen (HBsAg) positivity of 6 months and elevated HBV-DNA levels (>104 copies/mL), irrespective of the hepatitis B ‘e’ antigen status. Those already on treatment and those with an HIV or HCV co-infection or any other immunodeficiency state were excluded. Thus, a total of 60 patients were recruited. Patients were informed, and written consent taken before including them in the study.
Drugs given
Out of the 60 patients recruited, 21 of them were started on lamivudine plus adefovir combination (group A), 20 on entecavir monotherapy (group B), and 19 on tenofovir monotherapy (group C). Baseline data was compared between the 3 groups to ensure comparability. All patients were followed up for a period of 24 weeks. Biochemical, serological, and virological markers were measured at 12 and 24 weeks.
Biochemical markers
Serum bilirubin, alanine transaminases (ALT), and aspartate transaminases (AST) were measured using an automated biochemistry analyzer (HITACHI 917®, Roche, Germany) at baseline, 12, and 24 weeks.
Serological markers
On freshly collected samples, serum HBsAg assay was done using third generation HBsAg Uni-Form II kit (Hepanostika™, ELISA, bioMerieux, France, reference no. 280251) at baseline 12 and 24 weeks. Serum HBeAg and Anti-HBe levels were measured using VIDAS™ (bioMerieux, France. reference no. 30305) again at baseline 12 and 24 weeks. All tests were done as per the procedures strictly as described by the manufacturer.
Virological markers
HBV-DNA levels were measured by real time PCR (HBV Real-TM Quant SC kit, Biotron. Catalogue number. TV5-100/2FRT) with a linear range between 400 to 108 copies/ml at baseline 12 and 24 weeks. Our laboratory uses external quality assurance program with quality control in molecular diagnostics (QCMD), UK.
Statistical analysis
Quantitative data were analyzed by Kruskell Wallis test. Multiple comparisons were done by Wilcoxon rank-sum (Mann-Whitney) test with Bonferroni correction. For qualitative data, analysis was done using Fisher's exact or Pearson Chi-Square tests. Significance was defined as P < 0.05.
RESULTS
Patient data
A total of 60 patients were included in the study, of which 21 were put on lamivudine plus adefovir (group A), 20 on entecavir (group B), and 19 on tenofovir (group C), as shown in Table 1.

In group A, 90% (19/21) were males with the mean age of 38.38 ± 12.08. 10 patients (48%) were HBeAg-positive at baseline. The median HBV-DNA levels in this group were 5.71 log10 copies/mL (range 4.2 - 9.5 log10 copies/ml). Median aspartate transaminase, alanine transaminase and serum bilirubin levels were 52 (range 24 - 154 IU/mL), 53 (range 29 - 163 IU/mL), and 1.2 (range 0.4 - 3.2 IU/mL).
In group B, 80% (16/20) were males with the mean age of 42.15 ± 17.11. 15 patients (75%) were HBeAg-positive at baseline. The median HBV-DNA levels were 7.69 log10 copies/mL (range 4.0 - 8.5 log10 copies/ml). Median aspartate transaminase, alanine transaminase, and serum bilirubin levels were 58 (range 22 - 130 IU/mL), 44 (range 17 - 151 IU/mL), and 2 (range 0.5 - 4.9 IU/mL).
In group C, all (19/19) were males with the mean age of 34 ± 9.60. 10 patients (53%) were HBeAg-positive at baseline. The median HBV-DNA levels were 5.91 log10 copies/mL (range 4.0 - 10.1 log10 copies/ml). Median aspartate transaminase, alanine transaminase, and serum bilirubin levels were 59 (range 27 - 1490 IU/mL), 57 (range 25 - 1004 IU/mL), and 1.1 (range 0.4 - 31.5 IU/mL). The results show that baseline characteristics in all the 3 groups were similar, and the difference was statistically insignificant between the groups [Table 1].
Biochemical markers
The baseline characters were similar, and the difference was statistically insignificant between the groups. At baseline, 16/21, 13/20, and 16/19 patients were having elevated alanine transaminase levels in group A, B, and C of which 6 (38%), 4 (31%), and 4 (25%) patients respectively had their alanine transaminase levels normalized by 12 weeks of therapy and 9 (56%), 10 (77%), and 13 (81%) patients had their alanine transaminase levels normalized by 24 weeks of therapy [Table 2]. Among the 15/21 patients who had elevated aspartate transaminase levels in group A, 5 (33%) had their levels normalized by 12 weeks and 9 (60%) by 24 weeks. In group B, 14/20 patients had elevated aspartate transaminase levels, of which 5 (36%) and 10 (71%) had their levels normalized by 12 and 24 weeks, respectively. Of the 17/19 patients with elevated aspartate transaminase levels in group C, 6 (35%) and 13 (76%) patients had their levels normalized by 12 and 24 weeks, respectively [Table 2].
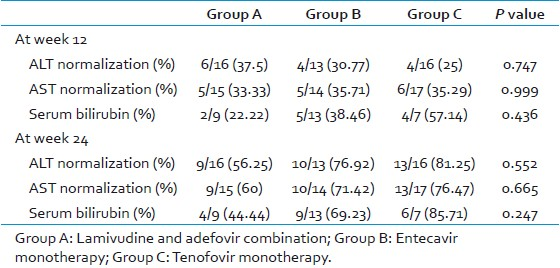
Nine patients (9/21) had elevated serum bilirubin levels in group A, of which 2 (22%) and 4 (44%) had their levels normalized by 12 and 24 weeks. In group B, 13/20 patients had elevated serum bilirubin levels at baseline, of which 5 (38%) had their levels normalized by 12 weeks of therapy and 9 (69%) had their values normalized by 24 weeks of therapy. Out of the 7/19 patients, who had elevated serum bilirubin levels in group C, 4 (57%) and 6 (86%) patients had their values normalized by 12 and 24 weeks of therapy. There was no significant difference between the 3 groups [Figure 1].
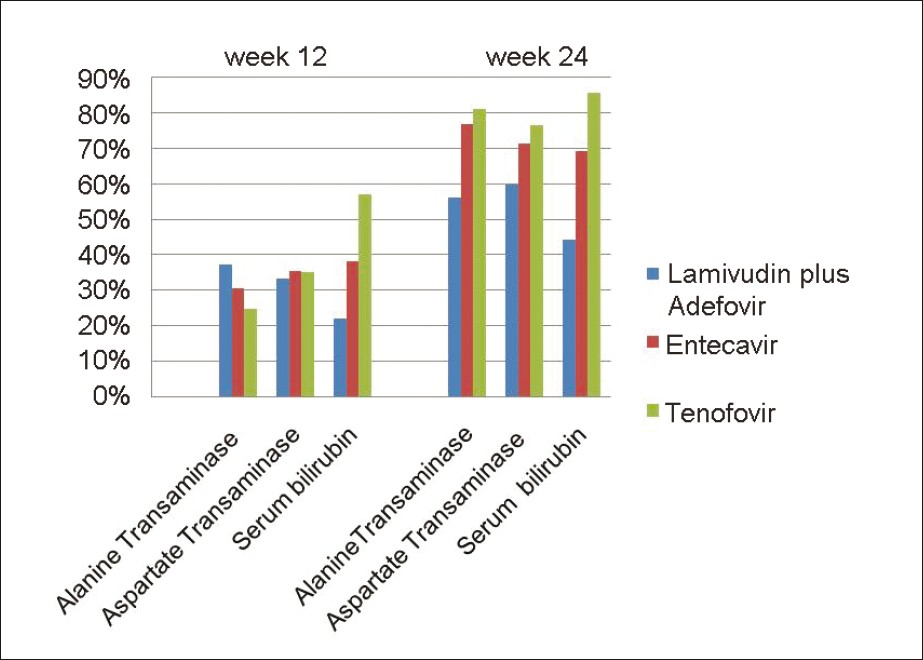
- Normalization of the biochemical markers in all the three groups at week 12 and 24. Columns represent the percentage of patients whose biochemical markers have normalized after treatment. The difference was not statistically significant between the groups
Serological markers
None of the patients became HBsAg-negative by 24 weeks in group A and B [Table 3]. One (5%) patient became HBsAg-negative in group C after 12 weeks of therapy, but it was not statistically significant (P-0.334). Three (10%) patients each in group A and C became HBeAg-negative after 12 weeks of therapy. None of the patients became HBeAg-negative after 12 weeks in group B. It was statistically significant (P-0.032). After 24 weeks of therapy, 7 (70%), 7 (47%), and 5 (50%) of patients became HBeAg-negative in group A, B, and C which was not statistically significant (P- 0.575) [Figure 2].
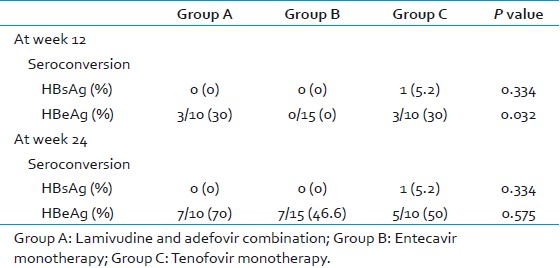
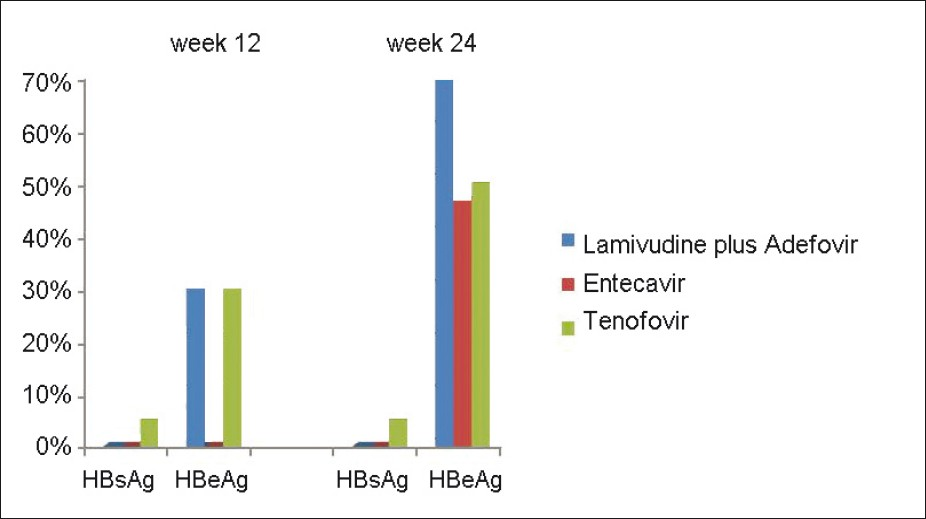
- HBsAg and HBeAg seroconversion in all the three groups at week 12 and 24. Columns represent the percentage of patients who have seroconverted* after treatment. The difference was not statistically significant between the groups. (*seroconversion here means conversion from positive to negative)
Virological markers
As mentioned above, the baseline virological markers were insignificantly different among the 3 groups. The number of patients who achieved complete clearance (undetectable levels) of HBV-DNA levels in group A, B, and C at 12 weeks were 0, 2 (10%), and 2 (11%), respectively [Table 4]. Similarly, at 24 weeks, these values were 4 (19%), 11 (55%), and 8 (42%), respectively. There was no significant difference between the groups at both 12 and 24 weeks (P-value 0.378 and 0.058, respectively) [Figure 3]. On follow up, the median decrease in HBV DNA levels in group A patients were 92.73% (range 23 - 99.78%) and 99.57% (range 32.2 - 100) at 12 and 24 weeks of therapy, considering the baseline HBV-DNA levels to be 100% [Table 4]. In group B, the median decrease was 99.74% (range 81.82 - 100%) and 100% (range 74.46 - 100%) at the same follow- up periods. In group C, this decrease was 97.19% (range 31.67 - 100%) and 99.99% (range 95.58 - 100 %), respectively. The differences were highly significant at both 12 (P-0.0036) and 24 (P-0.0125) weeks. At 12 weeks, the level of DNA decrease between group A and group B was highly significant (P-0.0007), whereas it was insignificant between group A vs. group C (P-0.1092) and group B vs. group C (P-0.1113). At 24 weeks, P-values were significant between group A and B (P-0.0115) and between group A and C (P-0.0084), whereas it was insignificant between group B and C (P-0.6431) [Figure 4].
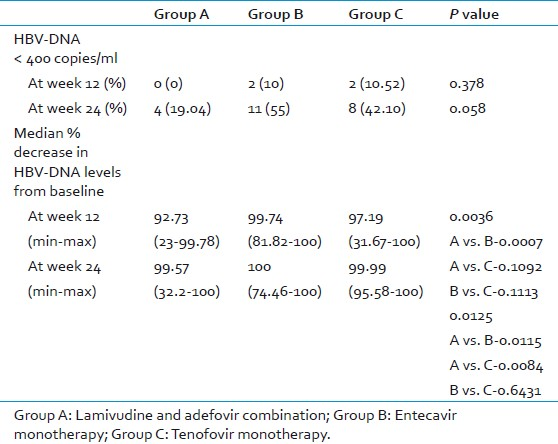
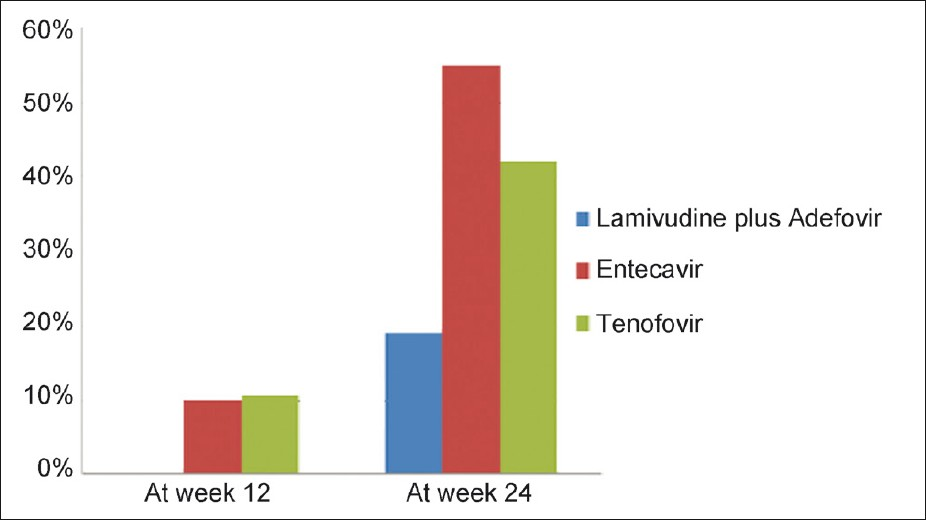
- Percentage of patients with HBV DNA levels <400 copies/mL (undetectable levels) in all the three groups at week 12 and 24. None of the patients had their HBV DNA levels reduced to undetectable levels in lamivudine and adefovir group. There was no statistically significant difference between the groups.
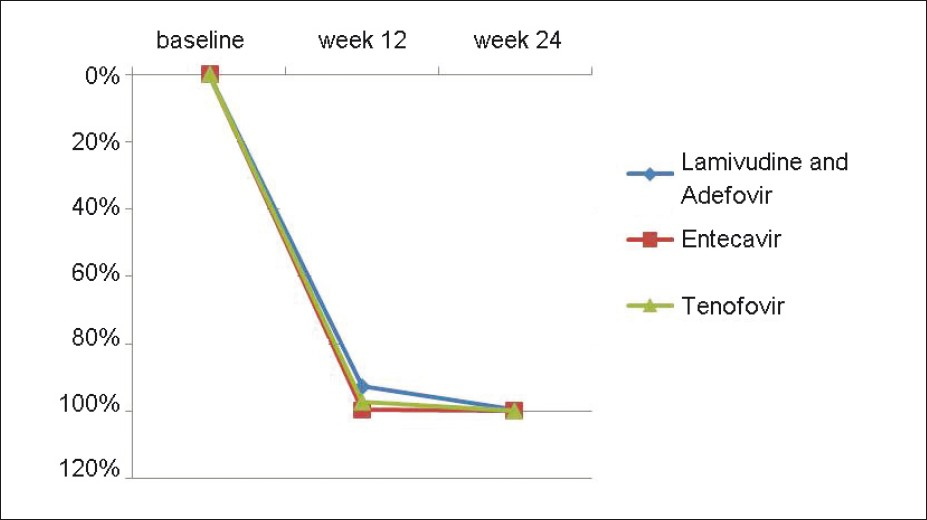
- Median % decrease of HBV DNA levels from baseline in all the three groups at week 12 and 24. In the lamivudine and adefovir, entecavir and tenofovir groups, the median % decrease in HBV DNA levels from baseline were 92.73, 99.74, 97.19 and 99.57, 100 and 99.99 at 12 and 24 weeks of therapy respectively and the difference was statistically significant (Table 4).
DISCUSSION
The main goal of treatment of chronic hepatitis B is to prevent complications and sequel of the infection, which includes cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC). To achieve this, profound and long term suppression of HBV-DNA is required. Though antivirals normalize the liver enzyme levels and help in seroconversion of HBeAg and HBsAg, persistent suppression of HBV-DNA levels are essential for prevention of complications. Current antivirals rarely eradicate HBV infection but can effectively suppress viral replication.[16]
Lamivudine and adefovir monotherapies are associated with a high degree of drug resistance. Lamivudine alone, if given, can have resistance in as many as 70% cases after 5 years.[17] In the past, many physicians preferred adefovir monotherapy as a second line drug for patients with lamivudine resistance. But, sequential monotherapies with these agents provide higher opportunity to the virus to develop multi-drug resistance.[16] Studies demonstrate that patients with lamivudine resistance are more likely to develop cross-resistance to adefovir if given as monotherapy, but showed lesser chances of developing resistance if given in combination.[18–20]
Clinical trials have shown that tenofovir effectively controls HBV replication in patients with both HBeAg-positive and HBeAg-negative disease, with efficacy levels of 75% and 93%, respectively.[21] Though mutation rtA194T has been implicated in conferring resistance, it is not so prevalent to avoid giving tenofovir monotherapy. Hence, tenofovir is a very good first line therapy option for both HBeAg-positive and HBeAg-negative patients. Similarly, entecavir can also serve the same purpose.
Entecavir has been known to suppress serum HBV-DNA to undetectable levels in 67%, 80%, and 82% of patients after 1, 2, and 3 years of therapy, respectively.[12] Since the development of resistance to entecavir requires mutation at 3 sites, the cumulative rate of emergence of resistance is very low at 1.2% even after 5 years of therapy.[1122–26]
Our study shows that entecavir monotherapy and tenofovir monotherapy are clearly better than lamivudine and adefovir combination in suppressing HBV-DNA replication. The median percentage decrease in HBV-DNA levels from baseline were 92.7%, 99.7%, and 97.2% respectively in group A, B, and C at 12 weeks of therapy, which is statistically highly significant (P-value-0.003). Also, at 24 weeks of therapy, the median percentage decrease of HBV-DNA levels were 99.5%, 100%, and 99.9% for group A, B, and C, which was again statistically significant (P-value-0.012). There was no statistically significant difference in the suppression of HBV-DNA levels between entecavir monotherapy and tenofovir monotherapy though clinically entecavir appeared to be better.
All the 3 groups showed no statistically significant difference in suppressing HBV-DNA to undetectable levels (<400 copies/ml) at 12 and 24 weeks of treatment (P-value 0.378 and 0.058). After 24 weeks of therapy, 19%, 55%, and 42% of patients had undetectable levels of HBV-DNA in group A, B, and C. Also, there was no significant difference in the seroconversion of HBsAg and HBeAg after 24 weeks in all the 3 groups (P-value 0.334 and 0.575, respectively) though at 12 weeks of therapy entecavir group showed no HBeAg seroconversion.
Even in the normalization of the liver enzymes ALT and AST and serum bilirubin levels, the 3 groups showed no statistically significant difference at 12 and 24 weeks of treatment.
From this study, though it was found that entecavir and tenofovir monotherapy are better than lamivudine and adefovir combination in suppressing HBV-DNA levels, there is no significant difference in the seroconversion of the viral markers and in normalization of biochemical markers in between the 3 groups. Also, clinically we found that those patients who respond to lamivudine and adefovir combination in the initial phase of treatment continue to respond to the therapy and those patients without any reduction in HBV-DNA levels at 12 weeks of therapy did not show any considerable difference after 24 weeks too.
Since entecavir and tenofovir are comparatively costlier drugs than lamivudine and adefovir, many of the patients are unable to afford for these drugs, which leads to incompliance in the treatment, especially among the lower socio-economic class followed by complications. Hence, for those who can afford for the costlier drugs, entecavir and tenofovir could be a better option as a first line therapy, but in those unable to afford for the same, lamivudine and adefovir combination can still be a good option.
In this study, we have followed up the patients through 24 weeks and hence changes in the response to these drugs in the long term could not be recorded. Also, a more sophisticated study with a larger sample size and longer period of follow-up has to be carried out, for which this study would serve as a base.
India is a country, which is classified as intermediately endemic for Hepatitis B.[27] With much of the patients belonging to lower socio-economic class, it is essential that we better concentrate on prevention and early diagnosis and treatment of the disease, which could significantly decrease the burden on this population.
ACKNOWLEDGEMENTS
We acknowledge Dr. Pratap Mouli (DM, Dept of Gastroenterology AIIMS) for all the support in the clinical aspect in this work. Our sincere thanks Mr. Ashish Upadhyay (Department of Biostatistics, AIIMS) for his valuable suggestions and analysis of the data. We thank our efficient technicians Mrs. Veena Balooni and Mrs. Shalini Singhal (Department of Laboratory medicine, AIIMS) for their technical help.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- The natural history of Hepatitis B virus infection. In: Lai CL, Locarnini S, eds. Hepatitis B virus. London: International Medical Press; 2002. p. :161-72.
- [Google Scholar]
- Hepadnaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, eds. Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier; 2005.
- [Google Scholar]
- Natural history of chronic Hepatitis B: Special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-52.
- [Google Scholar]
- Treatment of chronic hepatitis B with interferon-alpha: Cost-effectiveness in developing countries. Natl Med J India. 2002;15:320-7.
- [Google Scholar]
- Chronic Hepatitis (chapter 300) In: Harrison's Principles of Internal Medicine (17th ed). United states of America: McGraw-Hill companies; 2008. p. :1957-66.
- [Google Scholar]
- Impact of YMDD mutations during lamivudine therapy in patients with chronic Hepatitis B. Antivir Chem Chemother. 2001;12(Suppl 1):67-71.
- [Google Scholar]
- Virological response and hepatocarcinogenesis in lamivudine-resistant Hepatitis B virus genotype C patients treated with lamivudine plus adefovir dipivoxil. Intervirology. 2008;51:385-93.
- [Google Scholar]
- Meta-analysis: Adefovir dipivoxil in combination with lamivudine in patients with lamivudine-resistant Hepatitis B virus. Virol J. 2009;6:163.
- [Google Scholar]
- Combination of adefovir dipivoxil (ADV) and lamivudine (LAM) prevented emergence of ADV resistance mutations in chronic Hepatitis B (CHB) patients with LAM-resistant HBV. Gastroenterology. 2005;128:M945.
- [Google Scholar]
- Entecavir: A potent antiviral with minimal long-term resistance in nucleoside-naive chronic Hepatitis B patients. Expert Rev Anti Infect Ther. 2008;6:569-79.
- [Google Scholar]
- Clinical emergence of entecavir-resistant Hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob Agents Chemother. 2004;48:3498-507.
- [Google Scholar]
- Long-term Hepatitis B virus dynamics in HIV–Hepatitis B virus co-infected patients treated with tenofovir disoproxil fumarate. AIDS. 2005;19:907-15.
- [Google Scholar]
- Tenofovir for the treatment of Hepatitis B virus. Pharmacotherapy. 2009;29:1212-27.
- [Google Scholar]
- Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247-54.
- [Google Scholar]
- Chronic Hepatitis B virus infections in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-61.
- [Google Scholar]
- Monotherapy versus combination therapy for the treatment of chronic Hepatitis B. Expert Opin Investig Drugs. 2009;18:1655-66.
- [Google Scholar]
- Long-term safety of lamivudine treatment in patients with chronic Hepatitis B. Gastroenterology. 2003;125:1714-22.
- [Google Scholar]
- Emergence of adefovir-resistant mutants after reversion to YMDD wild-type in lamivudine-resistant patients receiving adefovir monotherapy. J Gastroenterol Hepatol. 2009;24:49-54.
- [Google Scholar]
- Dynamics of lamivudine-resistant Hepatitis B virus during adefovir monotherapy versus lamivudine plus adefovir combination therapy. J Med Virol. 2008;80:1160-70.
- [Google Scholar]
- Adefovir and lamivudine in combination compared with adefovir monotherapy in HBeAg-negative adults with chronic Hepatitis B virus infection and clinical or virologic resistance to lamivudine: A retrospective, multicenter, nonrandomized, open-label study. Clin Ther. 2008;30:317-23.
- [Google Scholar]
- Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic Hepatitis B. N Engl J Med. 2008;359:2442-55.
- [Google Scholar]
- Entecavir resistance is rare in nucleoside naive patients with Hepatitis B. Hepatology. 2006;44:1656-65.
- [Google Scholar]
- Long-term monitoring shows Hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-14.
- [Google Scholar]
- Stepwise process for the development of entecavir resistance in a chronic Hepatitis B virus infected patient. J Hepatol. 2007;46:531-8.
- [Google Scholar]
- Dynamics of Hepatitis B virus resistance to entecavir in a nucleoside/nucleotide-naive patient. Antiviral Res. 2009;81:180-3.
- [Google Scholar]
- Comprehensive evaluation of Hepatitis B virus reverse transcriptase substitutions associated with entecavir resistance. Hepatology. 2008;47:1473-82.
- [Google Scholar]
- Trend in seroprevalence of Hepatitis B virus infection among blood donors of coastal Karnataka, India. J Infect Dev Ctries. 2009;3:376-9.
- [Google Scholar]





