Translate this page into:
Metastasis of maxillary sinus adenoid cystic carcinoma to the humerus bone—A rare case report
*Corresponding author: Anurag Singh, MD, Department of Pathology, King George Medical University, Lucknow 226003, Uttar Pradesh, India anugsvm@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Sagar M, Singh A, Qayoom S, Singh US. Metastasis of maxillary sinus adenoid cystic carcinoma to the humerus bone—A rare case report. J Lab Physicians. 2024;16:219-22. doi: 10.1055/s-0043-1772851
Abstract
Adenoid cystic carcinoma accounts for 10 to 12% of all salivary gland malignant neoplasms with an estimated incidence of 3 to 4.5 cases per million per year. Perineural spread and multiple local recurrences are its defining features, although the liver and lung are the most frequent sites for distant metastases. It is extremely uncommon for adenoid cystic carcinoma of the maxillary sinus to spread to distant bones. Few cases of adenoid cystic carcinoma with distant bone metastasis have been reported. Here, we report a known case of the adenoid cystic carcinoma of the maxillary sinus in a 40-yearold male, with isolated metastasis to the left humerus bone presenting 4 years later to the excision of the primary lesion. The fine-needle aspiration cytology, trucut biopsy, and immunocytochemistry of the left humerus osteoexpansile lesion confirmed the diagnosis of metastatic adenoid cystic carcinoma. This rare case report re-emphasizes the distant metastatic potential of adenoid cystic carcinoma.
Keywords
Adenoid cystic carcinoma
humerus bone
maxillary sinus
metastasis
INTRODUCTION
Adenoid cystic carcinoma (ACC) accounts for 10 to 12% of all salivary gland malignant neoplasms with an estimated incidence of 3 to 4.5 cases per million per year.[1] Perineural spread and repeated local recurrences are the primary characteristics of ACC, while lung and liver are the most prevalent distant sites of metastases.[2] Remote bone metastases from ACC of the salivary gland are relatively uncommon.[3] Few reports of ACC with bone metastases have been recorded so far.[4,5] Here, we describe a case of a 40-year-old man who underwent excision of primary ACC of the left maxillary sinus and developed left humerus bone metastases 4 years later. The possibility for distant metastasis of ACC is re-emphasized by this unusual case report.
CASE REPORT
A 40-year-old man presented to the department of oncosurgery with chief complaints of left cheek swelling associated with severe pain, and reduced sensation over the left side of the face. He also complained of on and off fever and generalized weakness. There was no history of trauma or previous surgery. The plain and contrast-enhanced computed tomography (CT) scan of paranasal sinuses (PNS) and head showed a hypervascular, infiltrative mass measuring 5.5 × 3.8 × 3.2 cm, epicenter at maxillary sinus, and eroding the anterior wall of the maxilla, posterior maxillary sinus, posterolateral part of the hard palate, orbital floor, and adhered to periorbital tissue. No intraconal extension was noted.
Left total maxillectomy via mid-face degloving approach under general anesthesia was done with osteotomies over the maxilla along with medial, anterior, and posterior wall removal. On gross examination of resected specimen, infiltrative rubbery tan white mass was identified, measuring 4.5 × 3.0 × 2.5 cm extending close to the resected margins. The histopathology of the resected specimen was ordered that showed tumor disposed of in cribriform pattern and tubules filled with eosinophilic hyaline material. The tumor cells displayed round to oval hyperchromatic angulated nuclei, inconspicuous nucleoli, and scant amount of cytoplasm (Figure 1A, B). All the resection margins of the maxillectomy specimen were free from tumor invasion. Based on histomorphology, diagnosis of ACC was rendered. The postoperative plain CT of PNS was performed that showed no evidence of any enhancing lesion (Figure 1C).
![(A) Maxillary sinus tumor (hematoxylin and eosin [H&E] stain 40X). (B) Maxillary sinus tumor (H&E stain 400X). (C) Postoperative plain computed tomography of paranasal sinus.](/content/164/2024/16/2/img/JLP-16-219-g001.png)
- (A) Maxillary sinus tumor (hematoxylin and eosin [H&E] stain 40X). (B) Maxillary sinus tumor (H&E stain 400X). (C) Postoperative plain computed tomography of paranasal sinus.
The patient underwent a free flap repair and four stereotactic radiation sessions along with four cycles of platinum-based chemotherapy. After 4 years of initial diagnosis, he noticed a painful lumpinhislowerpartof leftarm (Figure 2A). The X-ray of the left arm was performed that showed an ill-defined enhancing osteoexpansile soft tissue lesion involving the distal shaft of left humerus (Figure 2B). Subsequently, fluorodeoxyglucose positron emission tomography-computed tomography whole body imaging displayed metabolically active bone and marrow lesionsinvolving the distal partof left humerus with the cortical breach. Noother metabolically active residual/recurrent disease is noted elsewhere in the body (Figure 2C).
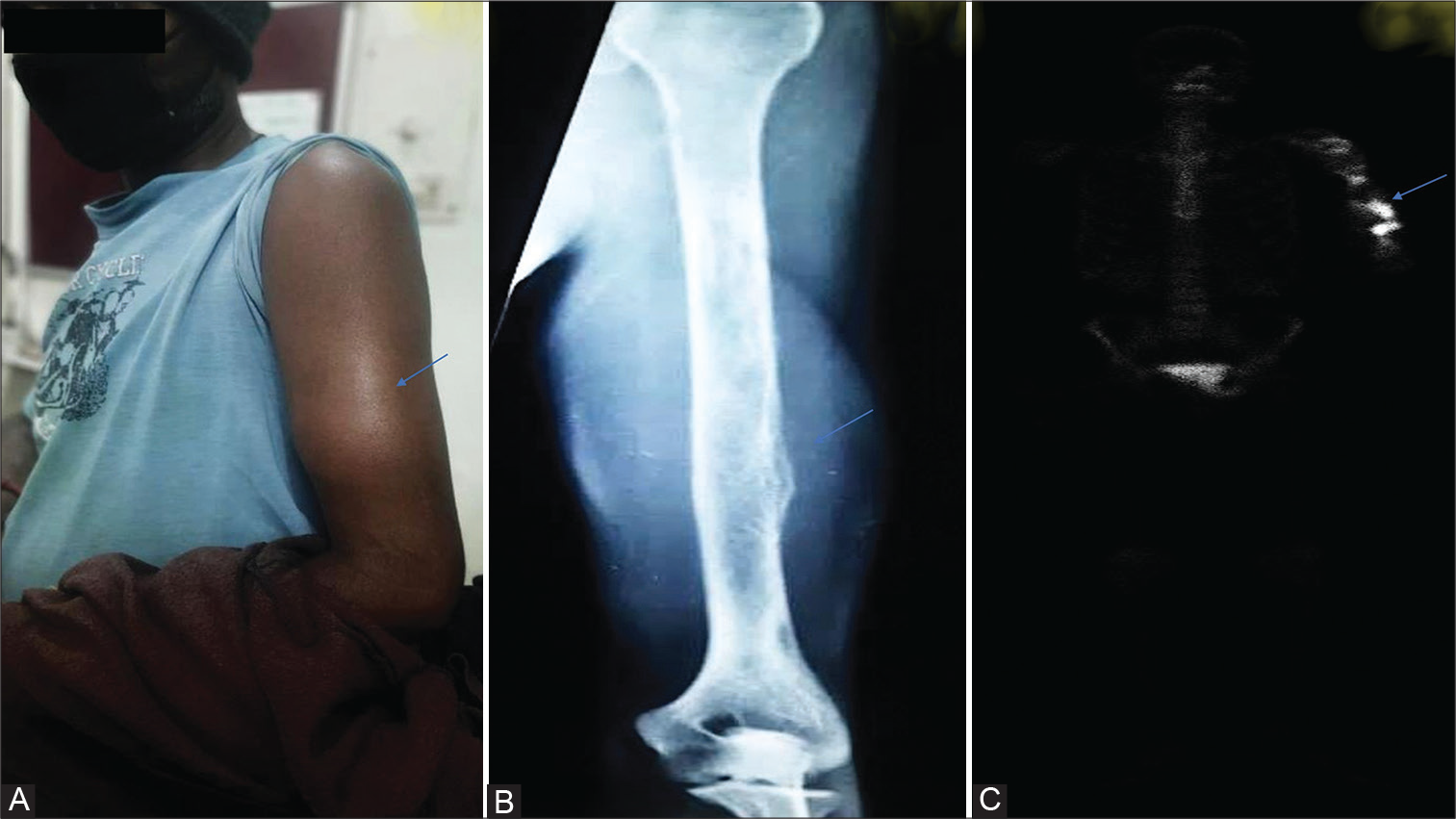
- (A) Clinical photograph of the patient. (B) X-ray of the left arm. (C) Fluorodeoxyglucose positron emission tomography-computed tomography whole body imaging.
The ultrasound-guided fine-needle aspiration cytology (FNAC) and trucut biopsy were performed from the left arm lump. The FNAC smears from left-hand swelling were cellular and showed papillaroid fragments of mild anisomorphic basaloid-type malignant epithelial cells on an eosinophilic hyaline background. These tumor cells displayed round to oval hyperchromatic nuclei, indistinct nucleoli, and scant cytoplasm (Figure 3A, B).
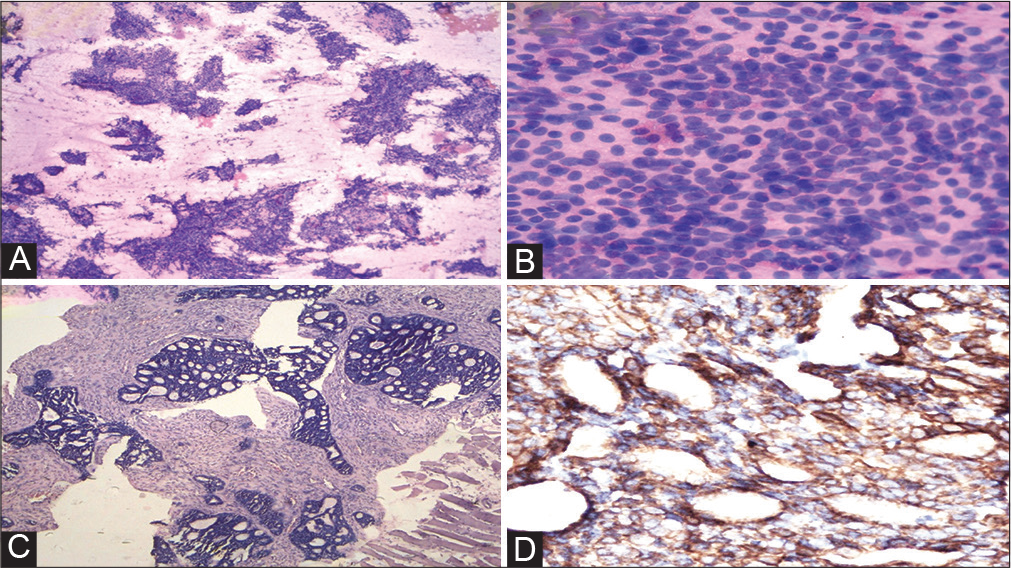
- (A) Fine-needle aspiration cytology (FNAC) from left-arm swelling (May Grunwald-Giemsa stain 100X). (B) FNAC from left-arm swelling (MGG stain 400X). (C) Trucut biopsy left arm (hematoxylin and eosin stain 40X). (D) Immunohistochemistry: anti-c-kit antibody (40X).
The section from trucut biopsy from the left arm lump also showed similar morphology of tumor cells as the primary lesion of the maxillary sinus (Figure 3C). Immunohistochemistry was also applied on trucut biopsy that displayed diffuse cytoplasmic expression for anti-ckit antibody in the tumor cells (Figure 3D). A definitive diagnosis of metastatic ACC to the left humerus (follow-up case of maxillary sinus ACC) was rendered. The patient had been planned for further management, but unfortunately, he succumbed to his illness after 3 months of detection of metastasis.
DISCUSSION
ACC of salivary gland is a relatively uncommon neoplasm. Only 3 to 5% of head and neck and 5 to 15% of sinonasal malignant neoplasms are ACC.[6] The patients’ age range for ACC is from 28 to 84 years with a median of 54.[5] years.[7] It can develop in various other organs, including the esophagus, uterine cervix, breast, lung, prostate, and tracheobronchial tree.[8] They spread most frequently to the liver and lungs. However, only 1.96% of metastases involve the bones, and metastases through the hematogenous route to distant bones are relatively rare.[9,10] Following bony metastases, there is a brief period of survival, often lasting 10 months.[11] Our patient died after 3 months of detection of bone metastases. Only a small number of cases of ACC with distant bone metastasis have been documented in the literature (Table 1).
| Sl. no. | Age/sex | Site of primary lesion | Site of distant metastasis | Duration between excision of primary lesion and distant metastasis | Treatment | Author, year |
|---|---|---|---|---|---|---|
| 1. | 62/F | Submandibular gland | Left big toe bone | 8 years | Surgery with radiotherapy | Zhang et al. 2019[4] |
| 2. | 52/M | Submandibular gland | Right toe | 12 years | Radiotherapy | Rafael et al. 2016[2] |
| 3. | 37/M | Left maxillary sinus | Lower spinal column and acetabulum | Presented with metastasis | Palliative radiation therapy along with adjunctive doxorubicin chemotherapy |
Lee et al. 2004[13] |
| 4. | 62/M | Submandibular gland | Intraspinal metastasis (L3-L4) |
15 years | Decompression with palliative radiotherapy |
Birkeland., 2003[14] |
| 5. | 52/M | Submaxillary gland | Left great toe | 8 months | NA | Weitzner 1975[5] |
| Index case | 40/M | Maxillary sinus | Left humerus bone | 4 years | Died before start of treatment | – |
Abbreviation: NA, not available.
A careful analysis of pertinent literature revealed that distant metastases to bone are incredibly uncommon. ACC spreads relentlessly to local and distant structures through direct extension or hematolymphatic pathway.[11] The majority of patients die 5 to 10 years after initial treatment, and it has a significant mortality rate. After 4 years of initial treatment, our patient also passed away with isolated bone metastasis.
There are no established treatment guidelines for cases of distant bone metastases in the postoperative cases of maxillary sinus ACC. Whenever there are superior alternatives to surgical resection, such as stereotactic radiation and platinum-based chemotherapy, they should be considered. In metastatic cases of ACC, targeted therapy such as anti-fibroblast growth factor, epidermal growth factor receptor, and tyrosine kinase inhibitors must be assessed because, as of yet, there is no long-term evidence to support their efficacy.[12]
CONCLUSIONS
ACC of the maxillary sinus with distant bone metastases is a very uncommon condition that requires case-specific treatment. Due to the few chemotherapeutic regimens available and the tendency for late metastases at distant sites, the prognosis for ACC with metastatic disease is extremely poor. This rare case report re-emphasizes the distant metastatic potential of ACC and hence such patients should be kept under regular follow-up for long periods.
Ethical Clearance
Approved.
Conflicts of Interest
None declared.
Financial Support and Sponsorship
None.
References
- Adenoid cystic carcinoma of head and neck. Am J Otolaryngol Head Neck Surg. 2018;1:1010.
- [Google Scholar]
- Adenoid cystic carcinoma of submandibular gland metastatic to great toes: case report and literature review. Clin Case Rep. 2016;4:820-823.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid cystic carcinomas of minor salivary glands. Auris Nasus Larynx. 2010;37:615-620.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasis of submandibular gland carcinoma to the toe bone: a case report. Br J Oral Maxillofac Surg. 2019;57:368-370.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid cystic carcinoma of submaxillary gland metastatic to great toe. Am Surg. 1975;41:655-658.
- [Google Scholar]
- Adenoid cystic carcinoma. An indolent but aggressive tumour. Part A: from aetiopathogenesis to diagnosis. Acta Otorhinolaryngol Ital. 2021;41:206-214.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid cystic carcinoma of intraoral minor salivary glands. Oral Oncol. 2008;44:1026-1031.
- [CrossRef] [PubMed] [Google Scholar]
- Adenoid cystic carcinoma of the head and neck-An update. Oral Oncol. 2015;51:652-661.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual case of exclusive liver metastases from adenoid cystic carcinoma of the submandibular gland: a role for surgery? Report of a case. Surg Today. 2011;41:596-599.
- [CrossRef] [PubMed] [Google Scholar]
- Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. 2002;24:779-783.
- [CrossRef] [PubMed] [Google Scholar]
- Gouri SRM Adenoid cystic carcinoma of the parotid metastasizing to liver: case report. Accessed August 16, 2023 at http://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-4-41
- [Google Scholar]
- Adenoid cystic carcinoma of the palate: case report and review of literature. Pan Afr Med J. 2016;24:106.
- [CrossRef] [Google Scholar]
- Sinonasal adenoid cystic carcinoma with widespread symptomatic bony metastasis. Ear Nose Throat J. 2004;83:271-273.
- [CrossRef] [Google Scholar]
- Spinal metastasis of submandibular gland adenoid cystic carcinoma: a case report. Surg Neurol. 2003;60:265-266.
- [CrossRef] [PubMed] [Google Scholar]





