Translate this page into:
Molecular Characterization of Methicillin-Resistant Staphylococcus aureus Causing Fatal Purulent Pericarditis
Address for correspondence: Dr. V Anil Kumar, E-mail: vanilkumar@aims.amrita.edu
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Though pericardial disease is common in patients with renal disease, purulent pericarditis is very rare. We report a fatal case of purulent pericarditis and sepsis due to methicillin-resistant Staphylococcus aureus in a 78-year-old male with systemic hypertension and renal disease along with the molecular characterization of its resistant mechanism.
Keywords
Methicillin-resistant Staphylococcus aureus
multi-drug resistant
purulent pericarditis
INTRODUCTION
Inflammation of the pericardium due to infection or non-infectious etiology is known as pericarditis. The most common forms are idiopathic and viral which are usually self-limiting and benign conditions. In contrast without treatment mortality in purulent pericarditis approaches 100%.[1] Predisposing factors include renal disease, previous cardiac and thoracic surgery, endoscopic oesophageal variecal sclerotherapy, laproscopic nissins fundoplication, fiberoptic bronchoscopy and percutaneous coronary angioplasty[2] The portal of entry is usually direct invasion of infection from adjacent pneumonia or empyema and to a lesser extent may be due to hematogenous seeding from a distant infection. We report a fatal case of acute purulent pericarditis due to methicillin-resistant Staphylococcus aureus (MRSA) with molecular characterization of its resistance mechanisms.
CASE REPORT
A 78-year-old male who was a known case of squamous cell carcinoma of buccal mucosa, which was excised with cervical lymph node dissection two years ago along with systemic hypertension and renal failure (Urea 179 mg/dl, Creatinine 5.28 mg/dl) was hospitalized with complaints of fever since 10 days and dyspnoea on exertion class III of two days duration. A chest X-ray [Figures 1 and 2] showed massive pericardial effusion and an electrocardiogram (ECG) [Figure 3] revealed low voltage waves, ST elevation, atrial fibrillation and a fast ventricular rate. The white cell count was 21 K/μl with 88% neutrophils and liver enzymes were also elevated (SGOT 103 IU/L, SGPT 80 IU/L). The patient developed cardiac tamponade. Emergency pericardiocentesis was done and 800ml of fluid was drained and a pigtail catheter was introduced through a subxiphoid incision and placed under fluoroscopic guidance in the pericardial cavity. Pericardial fluid analysis showed ADA 15.2 U/L, glucose 97.8 g/dl, protein 5.84 g/dl.
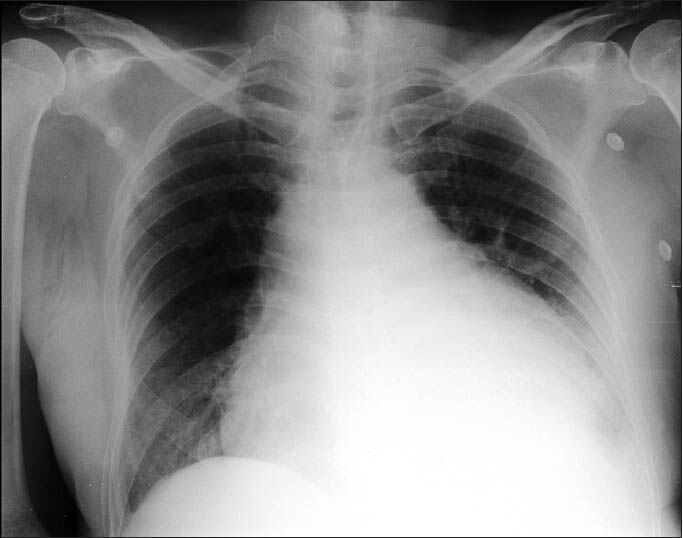
- Chest X-ray on admission showing a massive pericardial effusion with a pig-tail catheter in situ for drainage
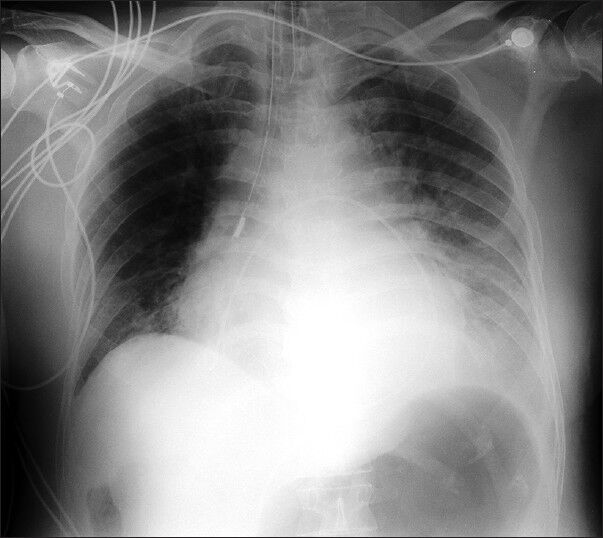
- Chest X-ray after pericardiocentesis showing a pig-tail catheter left in situ for continuous drainage
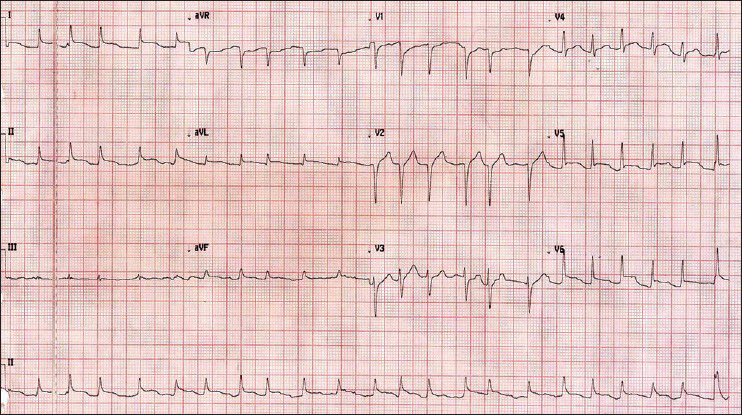
- Electrocardiogram on admission showing low voltage waves in leads II, III and aVF; ST elevation in leads I, II, aVL and chest leads V1 – V6; atrial fibrillation and a fast ventricular rate of 134 beats/min
Blood and pericardial fluid cultures grew Gram positive cocci which were identified as MRSA with the Vitek 2 (BioMerieux, Inc., Durham, NC). Both the MRSA isolates (from blood and pericardial fluid) were resistant to multiple classes of antibiotics namely oxacillin, penicillin, trimethoprim-sulfamethoxazole, gentamicin and ciprofloxacin while susceptible to vancomycin, clindamycin, tertracyline, linezolid, quinupristin/dalfopristin, nitrofurantoin, and rifampicin as determined by Vitek 2 system. Vancomycin (1 μg/ml) and daptomycin (0.94 μg/ml) susceptibility was confirmed by Etest (BioMerieux, Inc., Durham, NC). The isolate was intermediately sensitive to erythromycin (1 μg/ml) and a D-test for inducible clindamycin resistance was found to be negative. The MRSA isolate from blood and pericardial fluid were identical and identification was confirmed by amplifying the 16S rDNA gene of the genus Staphylococcus and sau gene specific for S. aureus.[3] A multiplex polymerase chain reaction (PCR) was done to simultaneously detect genes responsible for resistance to four classes of antibiotics and the isolate was positive for aac A-aph D, erm (C) and mecA encoding for resistance to aminoglycoside, erythromycin and oxacillin respectively.[3] The organism was susceptible to tetracycline and, therefore, tetK and tetM genes were not amplified.[3] All the primers were synthesized at Sigma Genosys, India.
The patient who was empirically put on piperacillin/tazobactum was shifted to vancomycin when cultures identified the isolate as MRSA on the third day. His renal function worsened and he was put on hemodialysis and mechanical ventilatory support. Subsequently he developed septic shock and metabolic acidosis and expired on the fourth day of admission due to heart failure.
DISCUSSION
Purulent pericarditis historically a disease of children and young adults has been a rare entity in the modern antibiotic era. Since 1945, the median age at the time of diagnosis has increased from 21 to 49 years.[4] The predisposing factors in the post antibiotic era were found to be an underlying non-infectious condition like thoracic surgery or chronic renal disease. Other predisposing factors are rheumatoid arthritis, malignancy, immunosupression, and alcohol abuse.[5] Pericardial diseases are more common in patients with renal disease and frequency has declined from 20%, which was 35 years ago, to 5% presently.[6] In most of these cases pericardial fluid did not show any evidence of infection, making purulent pericarditis a rare entity. Due to the absence of typical pericarditis features diagnosis of purulent pericarditis is often delayed. These patients typically present with an acute illness characterized by fever, chills, and tachycardia. Pericardial rub and chest pain are frequently not present and ECG may be normal in 35% of cases.[47] Primary site of infection is rarely pericardium and four pathways have been described for its spread to the pericardium namely direct extension of an intrathoracic process, penetrating injury to the chest wall, local extension and hematogenous spread.[1] Purulent pericarditis following percutaneous procedures is being increasingly reported and therefore these nosocomial infections are more likely to be caused by multi-drug resistant (MDR) pathogens.
Though the introduction of antibiotics has drastically reduced the incidence of purulent pericarditis in the last few decades, it is slowly reemerging due to the increased prevalence of MDR pathogens. S. aureus is the most common cause of purulent pericarditis,[2] and therefore reports of MRSA purulent pericarditis are on the rise.[89] Even though there are potent antibiotics to treat MRSA a delay in diagnosis often leads to high mortality. Our case highlights one such case of fatal purulent pericarditis caused by and MDR MRSA where early diagnosis and appropriate empirical antibiotic therapy could have resulted in a favorable outcome.
CONCLUSION
We suggest that acute purulent pericarditis with MDR MRSA should be considered in patients with renal disease.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- The changed spectrum of purulent pericarditis: An 86 year autopsy experience in 200 patients. Am J Med. 1977;63:666-73.
- [Google Scholar]
- Purulent pericarditis: Report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88:52-65.
- [Google Scholar]
- Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J Clin Microbiol. 2003;41:4089-94.
- [Google Scholar]
- Purulent pericarditis: Review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993;22:1661-5.
- [Google Scholar]
- Surgical treatment of purulent pericarditis in children. J Thorac Cardiovasc Surg. 1983;85:527-31.
- [Google Scholar]
- A case of myopericarditis in a patient with methicillin-resistant Staphylococcus aureus community-acquired pneumonia. Ann Acad Med Singapore. 2008;37:243-5.
- [Google Scholar]
- Four cases of community-associated methicillin-resistant Staphylococcus aureus pericarditis. Infect Dis Clin Pract (Baltim Md). 2010;18:251-2.
- [Google Scholar]





